Vitalstar has been engaged in liver disease research for over two decades, establishing techniques for transplanting primary human hepatocytes (PHH), cultured or ex vivo expanded human hepatocytes, as well as induced differentiation hepatocyte-like cells into the immunodeficient mouse models URG and NPG-Fah, which can be induced liver injury, bath developed by ourself. By combining surgical methods with an optimized liver injury induction protocol, perioperative care, and detection and analysis methods, we have obtained highly humanized chimeric liver mice with a human hepatocyte replacement rate of over 90%. We can provide technical services for establishment of humanized liver mouse models. In addition, Vitalstar has also established techniques for transplanting congenic mouse hepatocytes or hepatocyte-like cells into B6-Fah mice and can provide technical services for this. The services Vitalstar can provide include:
1. Transplantation of Primary or ex vivo expanded human hepatocytes as well as directly differentiated hepatocyte-like cells.
2. Transplantation of rodent or other animal hepatocytes.
Case I: Validation of the repopulation capacity of differentiated hepatocytes (EPS-Heps) using URG® model[1].
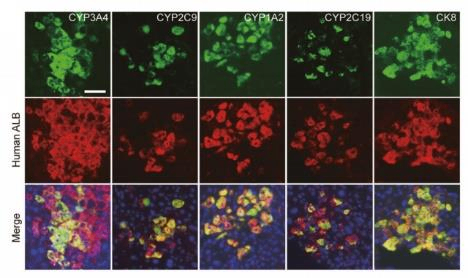
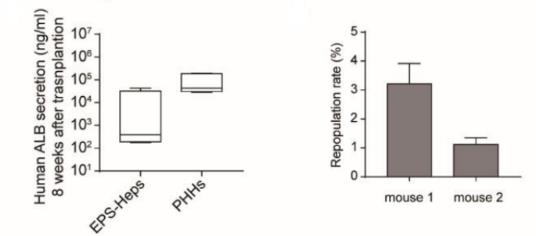
Fig 1. Repopulation of EPS-Heps and PHHs in URG® mice 8 weeks post transplantation.
After transplanting EPS-Heps into URG® mice for 8 weeks, the co-expression of human CYP3A4, CYP2C9, CYP1A2, and CYP2C19 with human ALB were detected on liver sections, and the secretion of human ALB was also found in the serum. These data indicate that EPS-Heps are liver-like cells with functionally mature characteristics that can reconstruct the damaged livers of mice.
Case II:Using URG® mouse model to study the treatment effect of transplantation of hepatocyte-like cells (HLC) on liver failure [2].
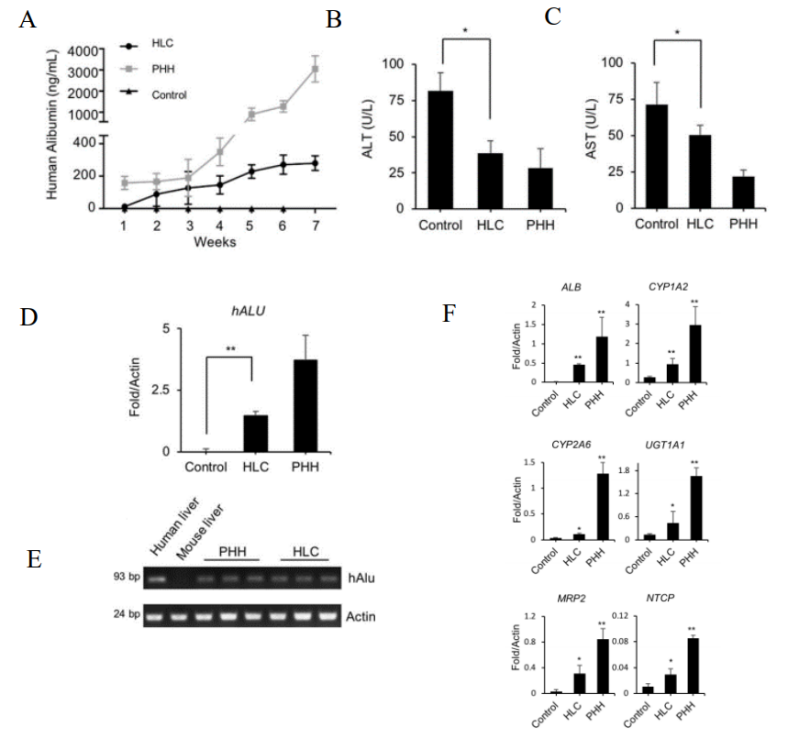
Fig 2. Reconstruction of liver in URG® mice after transplantation of HLCs.
8 weeks old male URG mice (n=5/group) were administered 15 mg/kg body weight of doxycycline (Dox) via intraperitoneal injection 12 hours before cell transplantation, with PBS as a control. Hepatocyte-like cells (HLCs) and primary human hepatocytes (PHHs) were transplanted into mice via splenic injection. After cell transplantation, URG® mice were continuously fed with Dox-containing drinking water for 6 weeks. Blood was collected from the tail vein weekly, and human albumin (ALB) levels were quantitatively measured by ELISA. The mice were euthanized at week 7. The results showed that after the transplantation of HLCs, the secretion of human ALB in the serum of URG® mice gradually increased. At 7 weeks, the serum concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) became nearly normal. Real-time PCR of the human-specific ALU sequence further confirmed the colonization of HLCs in the livers of the mice. The expression of human-specific genes such as ALB, and human hepatocyte-specific metabolic genes of phase I enzymes CYP1A2 and CYP2A6 in the chimeric liver tissue was also detected. These data indicate that HLCs can integrate into the livers of URG® mice and improve liver dysfunction.
Case III: Using URG® mice to evaluate the in vivo function of in vitro trans-differentiated hepatocytes[3].
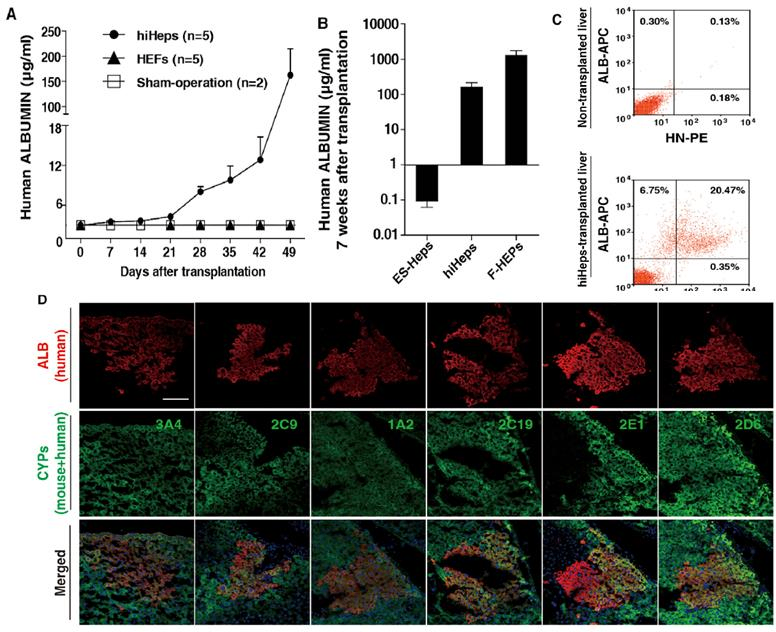
Fig 3. Reconstruction of liver in URG® mice after transplantation of human induced hepatocytes (hiHeps).
Intrasplenic injection of ES-Heps (n=16), hiHeps (n=5), and F-HEPs (n=6) was performed in URG® mice. Blood samples were collected, and human albumin (ALB) content was quantified using an ELISA assay. Liver sections were stained with fluorescent antibodies, and flow cytometry analysis was also performed. The results indicated that after transplantation of hiHeps, the secretion of human albumin in the mouse serum gradually increased. At six weeks post-transplantation, clusters of human ALB-expressing cells were observed in the livers of the transplanted mice. The metabolic functions of hiHeps in vivo were also confirmed, with the expression of CYP3A4, CYP2C9, and others analyzed. This demonstrated that hiHeps can stably grow and function in vivo. The study suggests that URG® mice can be used to validate hiHeps as a potential cell source for hepatocyte transplantation.
Vitalstar’s advantages:
(1) The advantage of URG® mice is that they do not express uPA when not induced, allowing for healthy growth of the mice; when induced with Dox, the Tet-On system drives the expression of uPA in the liver, leading to liver damage, at which point the mice can accept the engraftment of exogenous hepatocytes. Human hepatocyte replacement rate can achieve up to 90% of chimeric livers in URG® mice, and the transplantation window is not restricted.
(2) In comparison with other chimeric liver mouse model, the exogenous hepatocytes repopulating URG® mice need a shorter period than Fah-knockout mice (URG requires 6 to 9 weeks, while Fah KO mice require 3 to 6 months), therefore saving experimental time. Compared to mice with constant uPA expression, URG® mice have a larger body weight, with adult males weighing 31±3 g, and maintaining a weight of 25±3 g after hepatocyte transplantation. Therefore, Hu-URG® mice with highly humanized livers are in better healthy state and have better tolerance for experimental manipulations.
References:
1. Wang Q, Sun D, Liang Z, et al. Generation of human hepatocytes from extended pluripotent stem cells. Cell Res. 2020, 30:810-813.
2. Li Z, Wu J, Wang L, et al. Generation of qualified clinical-grade functional hepatocytes from human embryonic stem cells in chemically defined conditions. Cell Death Dis. 2019, 10:763.
3. Du Y, Wang J, Jia J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell. 2014, 14:394-403.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167