General Information
Strain Name | HuPBMC-DK-NPG |
Origin | Beijing Vitalstar Biotechnology Co., Ltd. |
Background | NPG |
Coat Color | Albino |
Development
DK-NPG mice combine severe combined immunodeficiency mutation (scid), Il2rγ knockout features, and highly immunodeficient characteristics. In order to slow down the GvHD response and prolong the experimental window, we deleted all IA, H2D (MHC class I molecules) and H2K genes (MHC class II molecules) from chromosome 17 of NPG mice based on the CRISPR-Cas9 technology, and obtained the MHC I/II double knockout mice (DK-NPG).
Considering that GvHD is mainly mediated by MHC class I and II molecules, and that class I molecules are more widely expressed, DK-NPG knockout as well as MHC class II molecules make it impossible for immune cells, such as transplanted Hu-PBMCs or isolated T cells, to recognize MHCs on the surface of the mouse cells, attenuate the immune attack, and slow down the occurrence of GvHD.
Phenotype
1. Reconstitution of human PBMC transplanted in DK-NPG mice
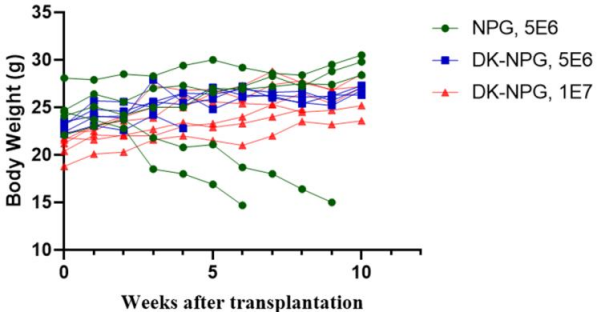
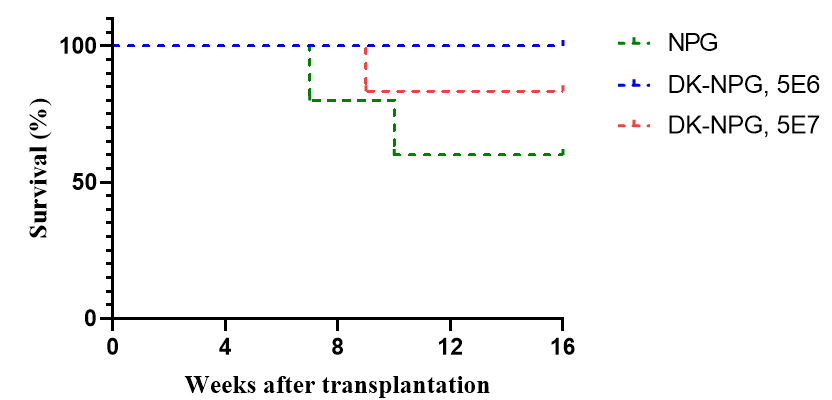
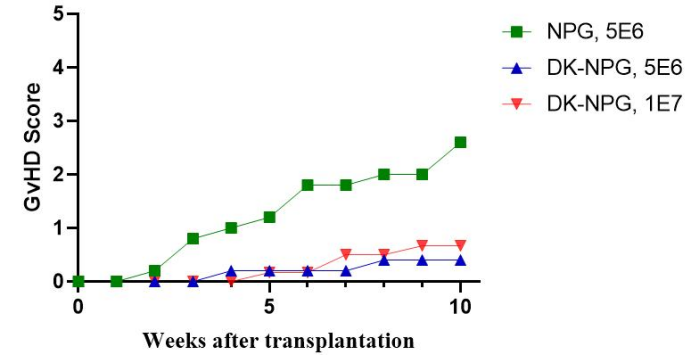
Fig1. Survival, body weight curves, and GVHD scores of NPG and DK-NPG mice inoculated with Hu-PBMC (5×106 vs. 1×107cells/mouse)
After NPG and DK-NPG transplantation with Hu-PBMC cells, DK-NPG mice had a longer survival cycle, steadily gained body weight and exhibited less severe GvHD symptoms (body weight, mobility, posture, hair and skin integrity, etc.) compared to NPG mice.
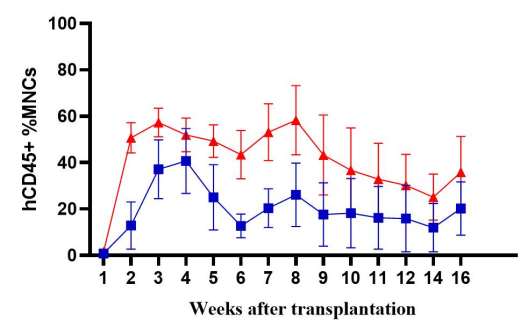
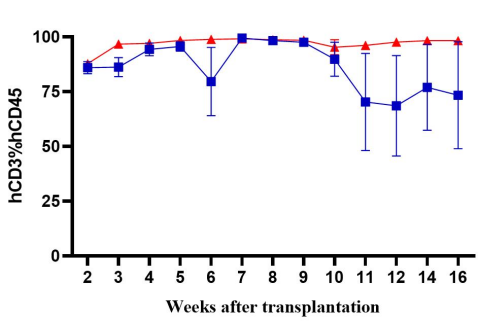
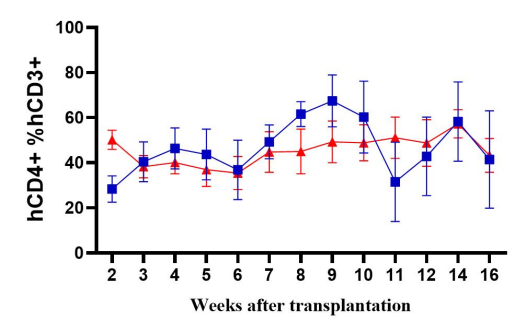
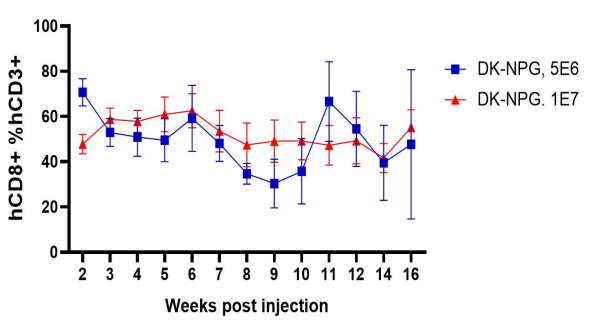
Fig2. Immune cell reconstruction efficiency in NPG and DK-NPG mice after inoculation with Hu-PBMC
DK-NPG rebuilt the human immune system well after inoculation with different doses of Hu-PBMC, and the trends were basically the same, indicating that the DK-NPG model stability and rebuilding efficiency were quite high. The reconstruction efficiency of 1E7 Hu-PBMC could reach 60%, and the experimental window could reach 14 weeks; the main reconstructed cells were human CD3 T cells, and the main differentiated hCD3 T cell populations were hCD4 and hCD8; this model could be used to evaluate the function of different CD3 T cell subsets, and to explore their roles in the development of GvHD.
2. Reconstitution effects of PBMC from different Donor sources in DK-NPG mice
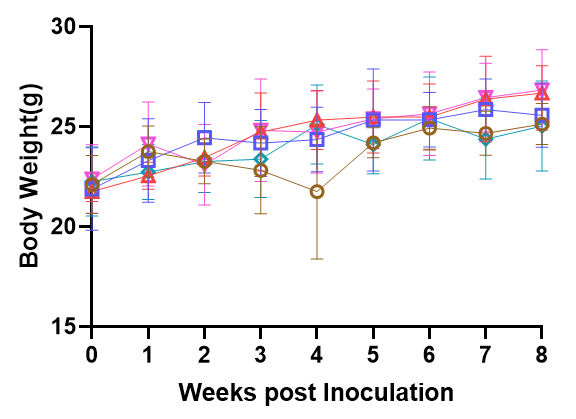
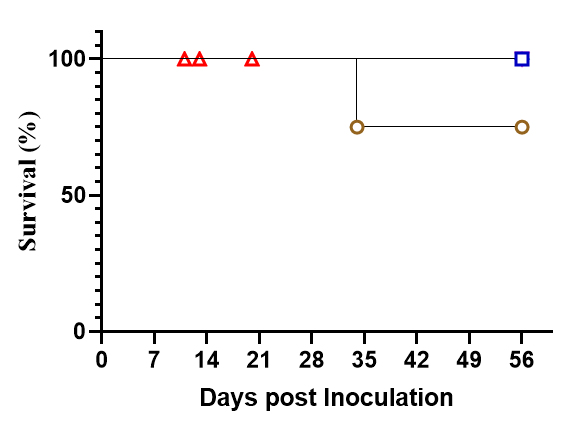
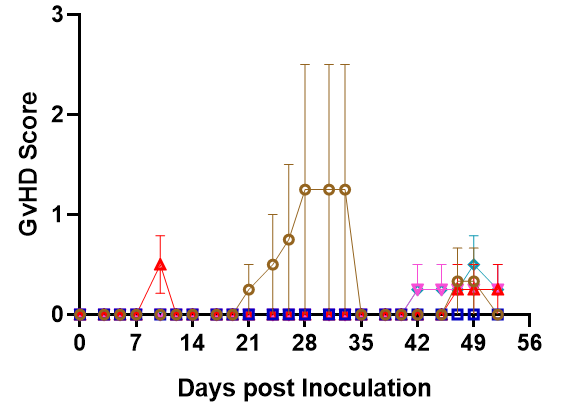
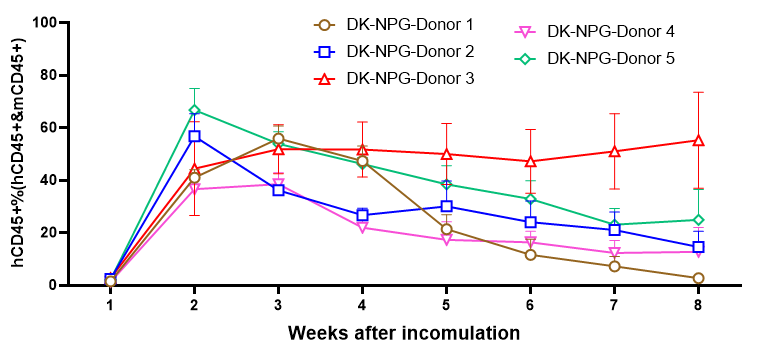
Fig3. Survival and efficiency of immune cell reconstitution in NPG and DK-NPG mice inoculated with Hu-PBMC of different donors.
DK-NPG and NPG inoculated with different doses of Hu-PBMC from different Donor, with more stable body weights, weaker degree of GVHD, and delayed onset of GVHD response in HuPBMC-DK-NPG than in PBMC-NPG.
Examples of different tumor modeling and efficacy studies in HuPBMC-DK-NPG mice
1. Constructing a human malignant melanoma model and evaluating the therapeutic effect of PD-1 antibody
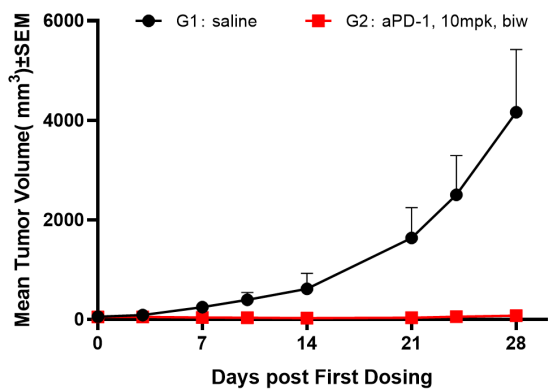
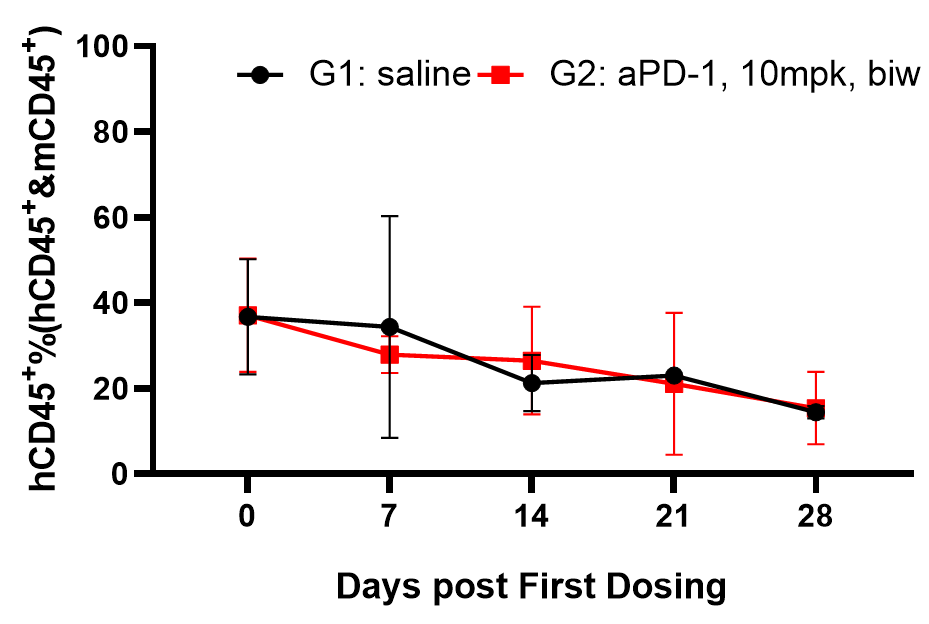
Fig4. Efficacy evaluation of PD-1 antibody in DK-NPG mice after Hu-PBMC reconstruction
Female NPG mice aged 5-6 weeks were inoculated with human PBMCs. One week after reconstitution, A-375 cells were subcutaneously inoculated. When the tumors grew to approximately 100-200 mm3, the mice were randomly divided into two groups (n=6 per group) and treated with PD-1 antibody. The levels of hCD45+ were continuously monitored. The results showed that the PD-1 antibody significantly inhibited the growth of tumor in DK-NPG mice.
2. Constructing a human cervical cancer model and evaluating the therapeutic effect of PD-1 antibody
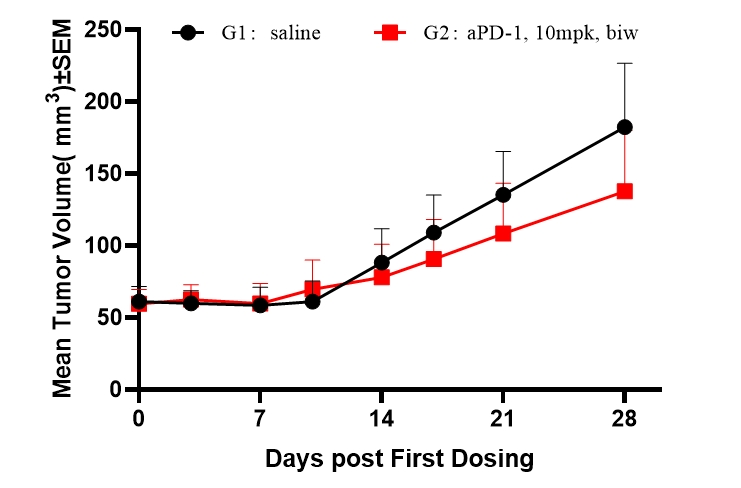
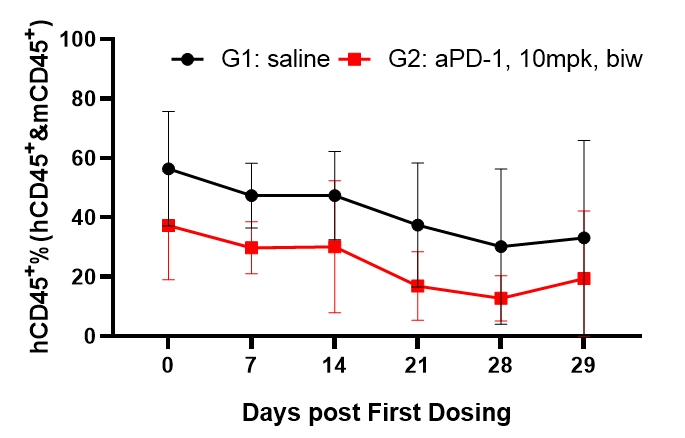
Fig5. Efficacy evaluation of PD-1 antibody in DK-NPG mice after Hu-PBMC reconstruction
5-6 week-old female NPG mice were inoculated with human PBMCs. One week after reconstitution, Hela cells were subcutaneously inoculated. When the tumors grew to approximately 60-80 mm3, the mice were randomly divided into two groups (n=6 per group) and treated with aPD-1 antibody. The levels of hCD45+ were continuously monitored. The results found that the aPD-1 antibody had a certain inhibitory effect on the growth of Hela tumors in DK-NPG mice.
HuPBMC-DK-NPG Mice Applications
1. Study the in vivo mechanism of GVHD in xenografts
2. Study of the activation, proliferation and function of CD3 T cells and differentiated CD3 T cell subsets, and exploration of their role in the development of GvHD
3. Pharmacodynamic evaluation, research and development of novel combinations of tumor immunity drugs, CAR-T, TCR-T, small molecule inhibitors, immune checkpoint blockade (ICB) and molecularly-targeted drugs targeting a variety of CD3 T cells, and assessment of the efficacy and safety evaluation of antibody drugs
4. Assessment of treatment-related cytokine release syndrome and evaluation of long-term toxicity related studies of cell therapy
5. Development of new humanized animal models
Reference
1. Ashizawa T, et al. Antitumor Effect of Programmed Death-1 (PD-1) Blockade in Humanized the NOG-MHC Double Knockout Mouse. Clin Cancer Res. 2017, 23(1):149-158.
2. Ashizawa T, et al. Impact of combination therapy with anti-PD-1 blockade and a STAT3 inhibitor on the tumor-infiltrating lymphocyte status. Immunol Lett. 2019, 216:43-50.
3. Santi Suryani Chen, et al. NCG-MHC-dKO mice - an excellent model for PBMC reconstitution and pharmacodynamic evaluation in the absence of GvHD. J Immunol. 2023, 210 (1_Supplement): 89.22.
4. Covassin L, et al. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rγ(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Exp Immunol. 2013, 174(3):372-388.
5. Brehm MA, et al. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 2019, 33(3):3137-3151.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167