General information
Strain Name | NOD.Cg-Prkdcscid Il2rgtm1/Vst |
Common Name | NPG mice |
Origin | Beijing Vitalstar Biotechnology Co., Ltd. |
Background | NOD |
Coat color | Albino |
Gene targeting technology | ES cell-based targeting |
Edited gene | Il2rg |
Development
NPG mice(NOD.Cg-PrkdcscidIl2rgtm1/Vst) are severely immunodeficient mice independently developed by Beijing Vitalstar Biotechnology Co., Ltd. Details are as follows::
First, ES cell targeting was performed: Il2rg gene targeting vector was constructed, and positive target cells whose exon 3-8 of Il2rg gene was replaced with pGK-neo resistance sequence were screened on ES cells with B6/129 F1 background (Fig 1). Then, ES chimeric mice with Il2rg gene knockout were obtained by blastocyst injection. The Founder backcrossed with NOD-scid mice, and the screened positive offspring were backcrossed with NOD-scid mice again. Through 12 generations of backcross, Il2rg- male mice were selected to mate with Il2rg+/- female mice, and finally PrkdcscidIl2rg-/- mice were obtained. Subsequently, NOD.PrkdcscidIl2rg-/- mice were multiplied and produced in an inbred manner.
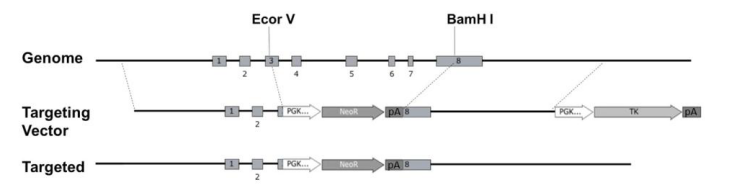
Fig 1. Schematic diagram of Il2rg gene targeting strategy
Genotype identification information
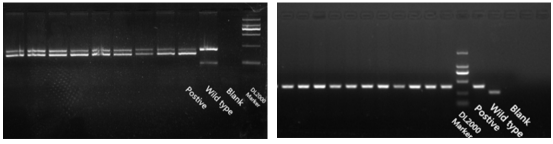
Fig 2. Prkdc gene PCR detection Fig 3. Il2rg gene PCR detection
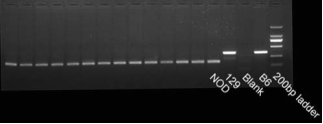
Fig 4. NOD background (Sirpa gene) detection
Phenotype
1. Growth curve
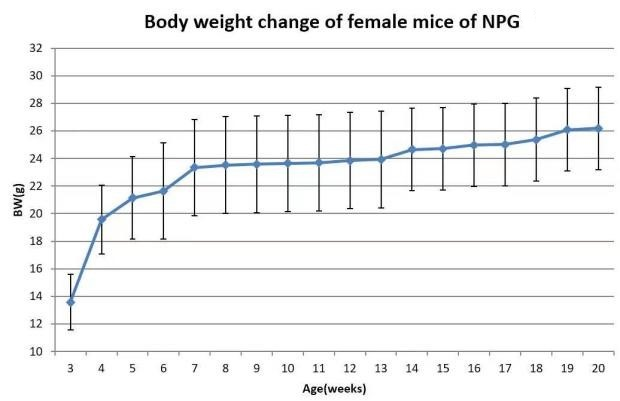
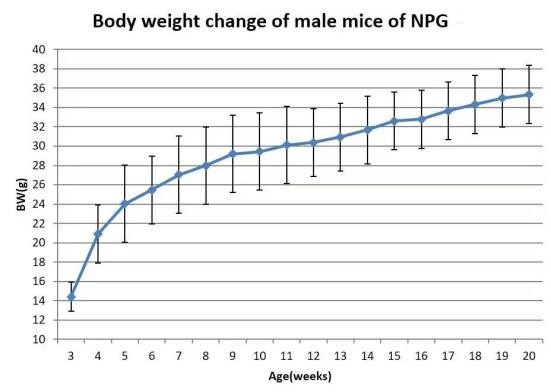
Fig 5. Body weight growth curve of NPG mice
2. Blood biochemical analysis
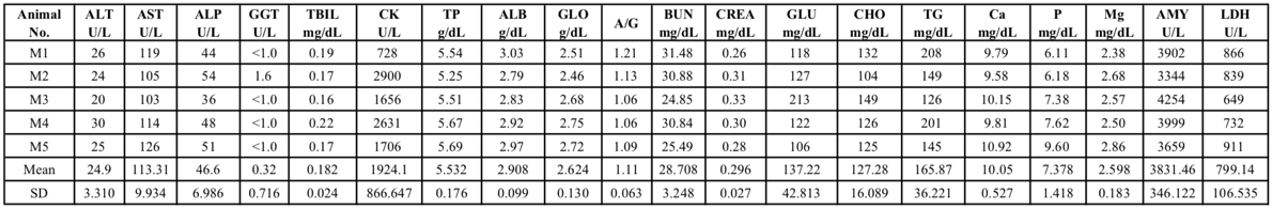
Tab 1. Blood biochemical analysis of 8-week-old NPG female mice
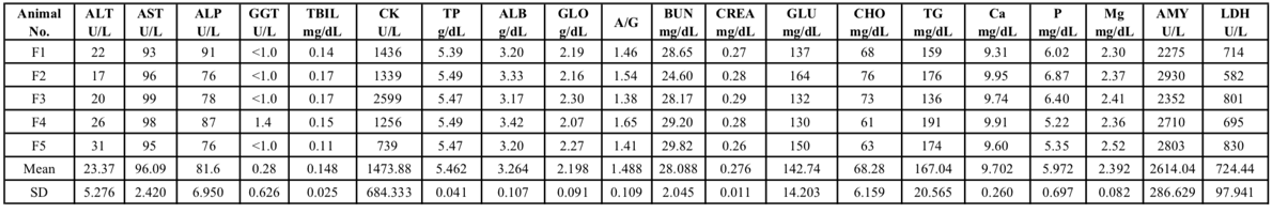
Tab 2. Blood biochemical analysis of 8-week-old NPG male mice
3. Analysis of immunocyte levels
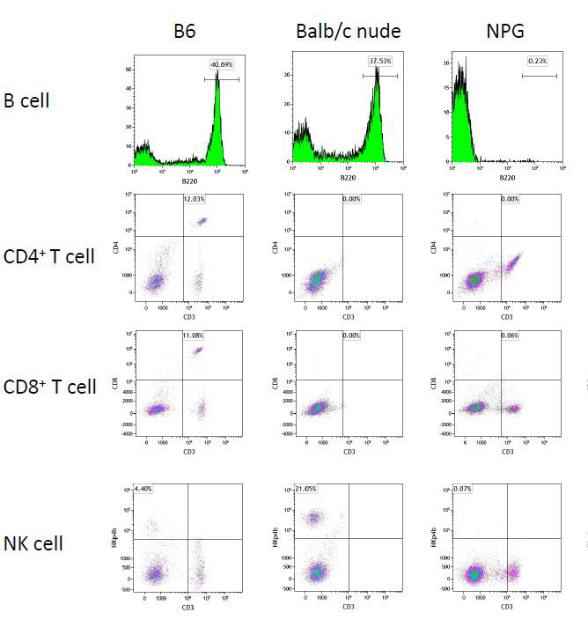
Fig 6. Detection of T, B, and NK cells in the peripheral blood of B6, Balb/c nude, and NPG mice
Results: Flow cytometric analysis indicates that, compared to B6 and Balb/c nude mice, there is a significant reduction in CD3+CD4+ and CD3+CD8+ T cells, B220+ B cells, and NKp46+ NK cells in the peripheral blood of NPG mice, to the extent that they are almost undetectable. This suggests that NPG mice are devoid of functional T, B, and NK cells, exhibiting the most severe degree of immunodeficiency.
4. Immunoglobulin analysis
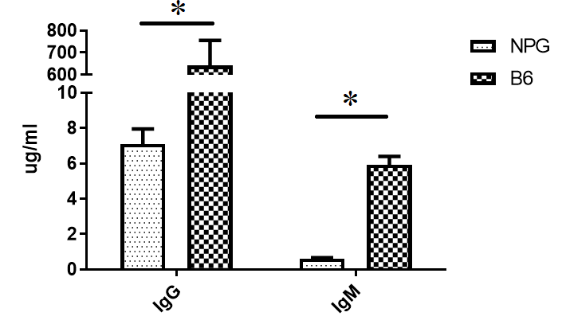
Fig7. Detection of immunoglobulin content in NPG, and B6 mice
The results indicate that the levels of IgG and IgM in the peripheral blood of NPG mice are significantly lower than those in B6 mice.
5. Orthotopic tumor model of NPG mice
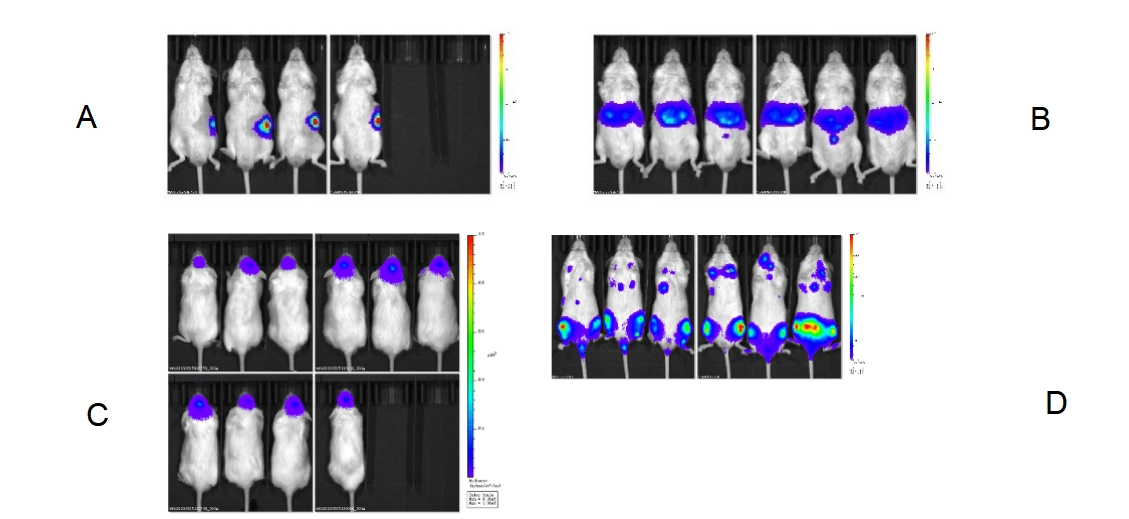
Fig 8. Diagram of orthotopic tumor of various organs in NPG mice. (A) Pancreas; (B) Splenic injection; (C) Intracranial; (D) Blood system
6. The efficiency of hematopoietic stem cell (HSC) transplantation and the reconstituted level of immune cell
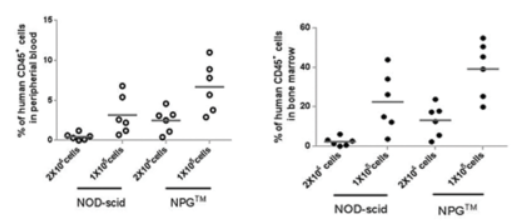
Fig9. NPG and NOD-scid mice were transplanted with human HSC, and the chimerism of hCD45+ cells was analyzed at 12 weeks post-transplantation
The results show that the immune cell reconstitution efficiency in NPG mice after HSC transplantation is significantly higher than that in NOD-scid mice.
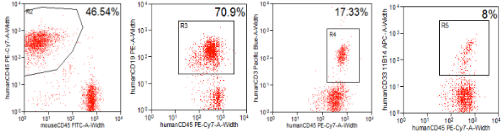
Fig10. High-level reconstitution of hematopoietic cell differentiation after transplantation of human HSCs into NPG mice
The results indicate that after transplanting 5×10^4 umbilical cord blood CD34+ cells for 16 weeks, high levels of human CD45+ cells, CD19+ B cells, CD3+ T cells, and CD33+ myeloid cells were detected in the peripheral blood of NPG mice.
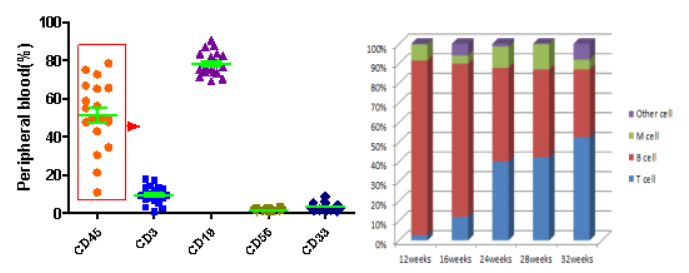
Fig 11. Reconstitution of various cell lineages in NPG mice following transplantation of human HSCs.
The results indicate that after the transplantation of umbilical cord blood CD34+ cells into NPG mice for 12 weeks and beyond, human-derived hematopoietic cells are stably reconstituted, with the proportion of T cells gradually increasing.
7. The level of reconstitution following peripheral blood mononuclear cell (PBMC) transplantation
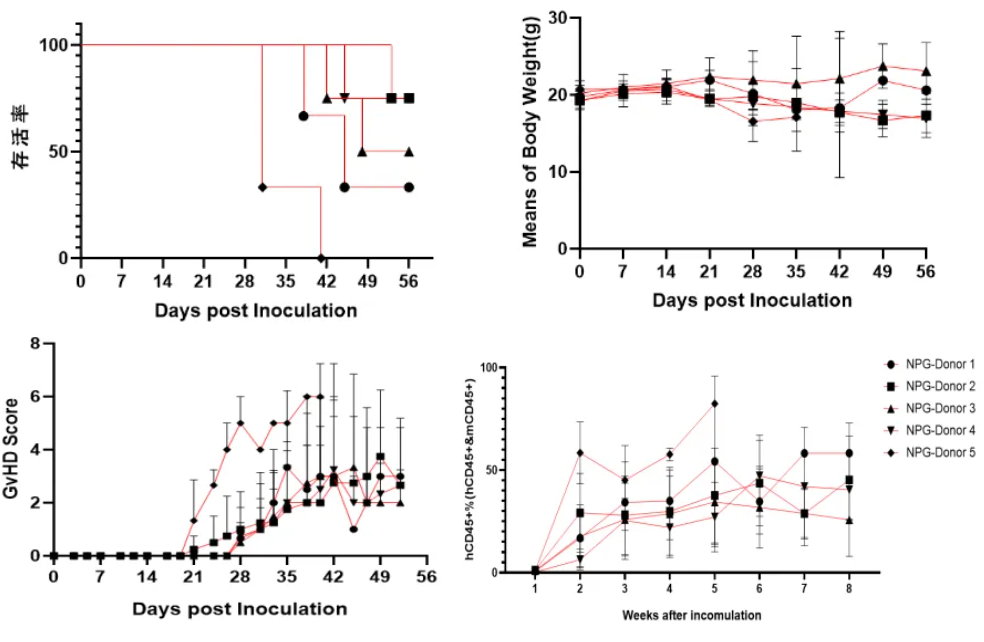
Fig 12. Survival rates, body weight, GvHD scores, and levels of reconstitution following transplantation of human PBMC from different donors (5×10^6).
NPG mice transplanted with various tumors and examples of efficacy studies
1. Constructing a human lymphoma model and the therapeutic effect of CD7-CAR-T cell treatment[1]
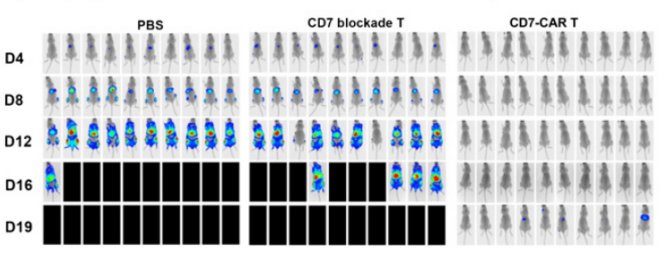
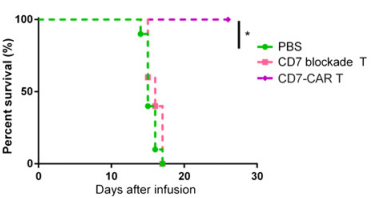
A B
Fig13. The antitumor effect of CD7 block CAR T cells in acute T lymphoblastic leukemia tumor models
6-8 weeks old female NPG mice were injected intravenously with 1×105 Luc+ GFP+ CCRF-CEM cells followed by a single intravenous injection of 5×108 cells/kg of CD7 block CAR T or CD7 blockade T cells 5 days later. PBS was used as a control. Result: CD7 blockade CAR T cells prevented fratricide and exerted potent cytolytic activity, significantly relieving leukemia progression and prolonged the median survival of mice.
2. Constructing a human lung adenocarcinoma model and the efficacy of bispecific antibody[2]
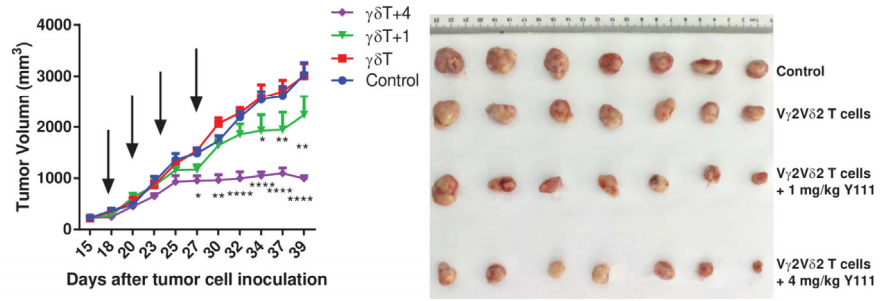
A B
Fig14. The combined usage of transfused Vγ2V2δ T cells with Y111 significantly inhibited tumor growth in vivo
6-8 week-old female NPG mice were s.c. inoculated with 5×106 H1975 NSCLC cells on Day 0. After seventeen days, mice were treated with i.v. transfused Vγ2Vδ2 T (1×106) cells w/wo 1 or 4 mg/kg Y111. These treatments were repeated twice per week for 2 weeks. Mice treated PBS were used as control. Results: Intravenous injection of Y111 (4 mg/kg) combined with Vγ2Vδ2 T cells significantly inhibited tumor growth in NPG mice.
3. Constructing a human prostate cancer model and evaluating the anti-tumor efficacy of plant proteins[3]
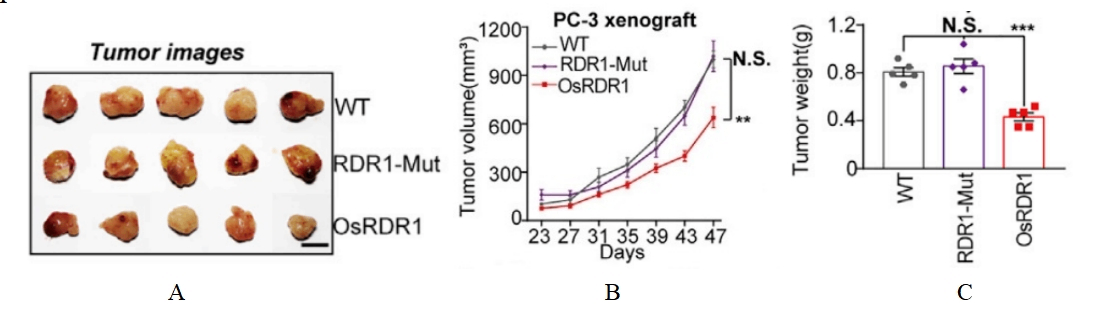
Fig15. The Anti-tumor effect of plant immunoglobulin RDR1
4-6 week-old female NPG mice (n=5 per group) were subcutaneously injected with RDR1-inducible 2×106 PC-3 cells in the abdominal area on day 0, and doxycycline (Dox) was administered in their drinking water to achieve ectopic expression of RDR1 to observe tumor growth. The results showed that wild-type, but not mutant, RDR1 significantly inhibited the size, volume, and weight of the resulting tumors.
4. Constructing a human pancreatic cancer model and evaluating the efficacy of NK cell treatment[4]
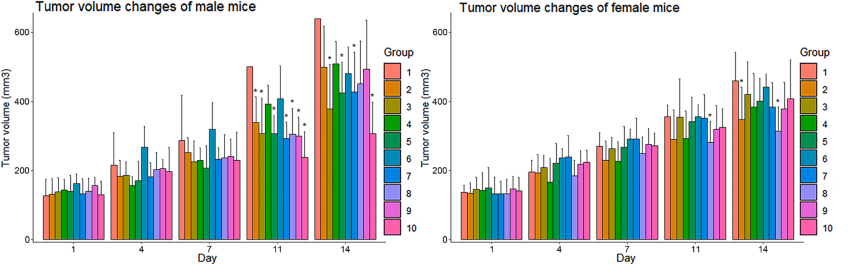
A B
Fig16. Tumor growth curve after NK cell therapy
7-8-week-old NPG mice (n=100, 50 male and female) were subcutaneously injected with 0.2 mL of logarithmic growth phase BxPC-3 cell suspension (2.75×107 cells/mL; cell viability of 97%) to establish a subcutaneous tumor-bearing mouse xenograft model. 8 days later, 10 groups were randomly divided into 10 groups according to sex, and the average tumor volume of each group ranged from 20-200 mm3, and different sites were injected with different numbers of NK cells. Group 1 were injected intravenously with sodium chloride as a carrier control, groups 2-4 were injected intravenously with human peripheral blood-derived NK cells, groups 5-7 were injected intraperitoneally with NK cells, and groups 8-10 were injected with intratumorally injected NK cells, and the therapeutic effects were observed. The results showed that intravenous injection was the safest method to give NK cells to mice carrying human PaC, and had obvious tumor suppression effects on tumors.
5. Constructing a human colorectal cancer model and evaluating the therapeutic effect of CAR-T[5]
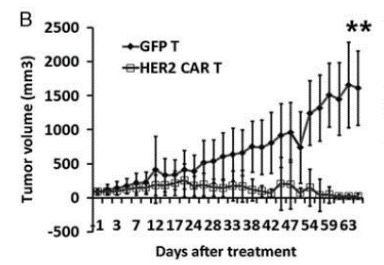
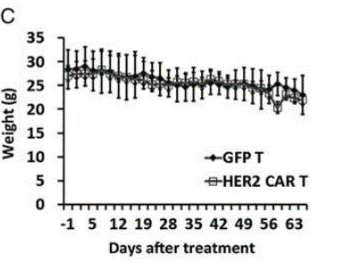
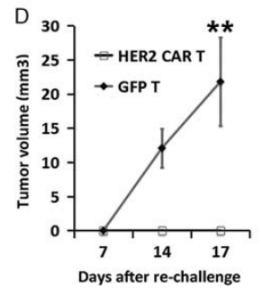
Fig17. Construction of NPG-PDX model and the anti-tumor effect of HER2 (Human Epidermal Growth Factor Receptor 2) specific CAR-T cells
6-10 week-old NPG mice (n=6 per group) were subcutaneously inoculated with tumor fragments (CRC) from colorectal cancer patients and infused with 5×106 T cells on days 7 and 14. P1 xenograft tumor tissues were re-transplanted on day 46. In this experiment, P1 and P2 generations of PDX models were used, and when the tumor volume reached 50-100 mm3, 2×106 GFP-T cells or HER2-specific CAR-T cells were intravenously injected into the tumor-bearing mice. The results showed that after 2 months of treatment with HER2-specific T cells, the tumors in NPG-PDX mice were completely eliminated, and the body weight growth parameters were not significantly different from those of the untreated control group.
6. Constructing a humanized mouse model of human gastric cancer and evaluating the therapeutic effect of PD-1 antibody[6]
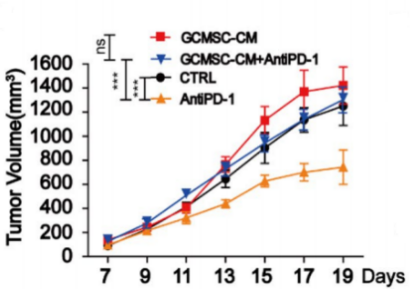
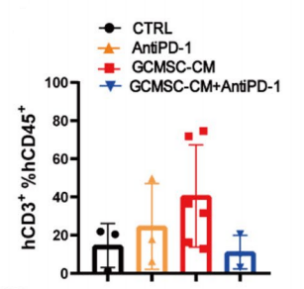

A B C D
Fig18. NPG mice transplanted with human umbilical cord blood CD34+ cells and inoculated with patient-derived gastric cancer tumors and tumor tissue immune cell levels
4-8 week-old NPG mice were irradiated with X-rays (1.2 Gy) and 24 hours later intravenously injected with 5×104 human umbilical cord blood CD34+ cells. After 12 weeks of reconstitution, gastric cancer cell lines were inoculated, and treated with GCMSC-CM and PD-1 antibody alone or in combination to observe tumor changes, and to detect the levels of CD45+CD3+, CD3+CD8+, and CD3+CD4+ in tumor tissues from different treatment groups. The results showed that after 21 days of treatment, PD-1 antibody therapy significantly inhibited gastric tumor growth, while the combination of GCMSC-CM with PD-1 antibody significantly increased tumor volume compared to PD-1 antibody alone, indicating that GCMSC-CM eliminated the inhibitory effect of PD-1 antibody on gastric tumor growth.
7. Human multiple myeloma model and efficacy research
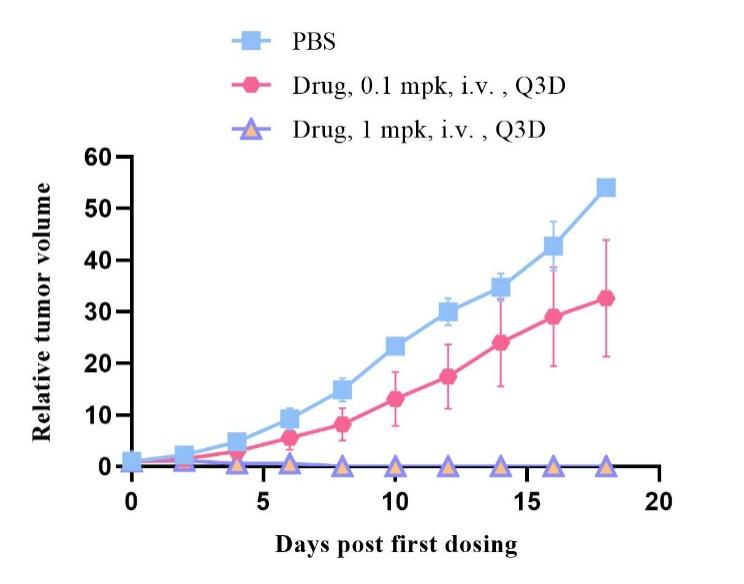
Fig19. Tumor growth curve after drug treatment
6-week-old NPG mice were transplanted with 5×106 human PBMCs, and one week later, H929 human multiple myeloma cells were injected subcutaneously. Different doses of drugs were administered intravenously via the tail vein for treatment studies. The results showed that the drug (at a dose of 1 mpk) had a significant inhibitory effect on the tumor.
NPG Mice Applications
1. Human cell or tissue transplantation (e.g. human colon cancer tissue)
2. Tumor and tumor stem cell research (e.g. human lymphoma, human pancreatic cancer)
3. ES and iPS cell research
4. Hematopoietic and immunology research
5. Human disease infection models (e.g. HIV infection)
6.Research and development of humanized animal models
Reference
1. Zhang M, et al. Autologous Nanobody-Derived Fratricide-Resistant CD7-CAR T-cell Therapy for Patients with Relapsed and Refractory T-cell Acute Lymphoblastic Leukemia/Lymphoma. Clin Cancer Res. 2022, 28(13):2830-2843.
2. Yang R, et al. Bispecific Antibody PD-L1 x CD3 Boosts the Anti-Tumor Potency of the Expanded Vγ2Vδ2 T Cells. Front Immunol. 2021,12:654080.
3. Qi Y, et al. A plant immune protein enables broad antitumor response by rescuing microRNA deficiency. Cell. 2022, 185(11):1888-1904.e24.
4. Huang L, et al. Acute toxicities of intravenous, intraperitoneal, or intratumoral injection of natural killer cells in human pancreatic adenocarcinoma-bearing mice: Randomized study. Int Immunopharmacol. 2023, 124(Pt A):110881.
5. Teng R, et al. Chimeric Antigen Receptor-modified T Cells Repressed Solid Tumors and Their Relapse in an Established Patient-derived Colon Carcinoma Xenograft Model. J Immunother. 2019, 42(2):33-42.
6. Huang C, et al. Gastric cancer mesenchymal stem cells via the CXCR2/HK2/PD-L1 pathway mediate immunosuppression. Gastric Cancer. 2023, 26(5):691-707.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167