Small nucleic acid drugs refer to drugs that can use small nucleic acid molecules such as siRNA, miRNA and antisense nucleic acid (ASO) to specifically silence the expression of disease genes in order to cure specific diseases. Including siRNA, miRNA, ASO, small activating RNA (saRNA), aptamer (aptamer), transfer RNA (tRNA) fragments, antibody nucleic acid-coupled drugs (ARC), etc. Due to its rich candidate targets, short research and development cycle, long-lasting efficacy, high specificity and high clinical development success rate, it is expected to become the "third pharmaceutical wave" after small molecule drugs and antibody drugs. Small nucleic acid drugs are mainly used in the treatment of tumors, a variety of rare diseases such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, spinal muscular atrophy, viral diseases, kidney diseases, cardiovascular diseases.
Based on the characteristics of preclinical research on small nucleic acid drugs, Vitalstar has developed a series of target genes humanized mice, humanized liver mice Hu-URG® and HBV-Tg mice suitable for the development of small nucleic acid drugs. At the same time, Vitalstar is equipped with a series of sampling and testing equipment to provide one-stop pre-clinical research services on small nucleic acid drugs. It is used for drug distribution, safety evaluation, pharmacological efficacy and other service systems to understand the potential role and safety range of small nucleic acid drugs in the human body. To help researchers optimize the design and development of small nucleic acid drugs, accelerate their entry into clinical trials, and improve their success in clinical applications.
(1) Drug distribution in the body’s tissues.
1. Experimental model
Hu-URG® humanized liver mice (which can express human liver-related genes and mimic human liver physiology and drug metabolism processes)
2. Experimental design
Determine the administration route and dose of small nucleic acid drugs, considering intravenous, intraperitoneal or oral administration. At the same time, appropriate time points were designed to collect animal samples in order to study the distribution dynamics of drugs at different time points. For example, the collection of animal blood samples and plasma separation for the analysis of blood drug concentration; Tissue samples (such as liver, kidney, heart, etc.) are homogenized or sliced to analyze the distribution of drugs in different tissues.
3. Detection indicators
Drug concentration analysis, such as drug time-concentration curve (PK curve); Drug distribution image analysis, the use of fluorescence imaging technology, directly observe and quantify the distribution of drugs in tissue sections or throughout the body; Impact of biomolecular markers: To analyze and evaluate the effect of small nucleic acid drugs on specific gene expression in liver or other tissues through real-time fluorescence quantitative PCR, Western blot or immunohistochemistry; Liver function testing, measuring serum liver function indicators, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), etc., to assess the potential impact of small nucleic acid drugs on liver function.
(2) non-GLP safety evaluation
1. Experimental model
C57BL/6J, C57BL/6N, humanized mice with drug target genes (hPCSK9, hGCGR, hGLP1R, hPD-1, hPD-L1, etc.), Hu-URG® and other mouse models.
2. Experimental design
Determine the route and dose of the small nucleic acid drug (single administration or continuous administration), considering intravenous, intraperitoneal or oral administration. Regularly observe and record the general health status and behavior changes of the animals, including food intake, weight changes, hair status, etc., to assess the impact of drugs on the overall health of the animals; Design appropriate time points to collect blood and tissue samples from animals to assess drug safety indicators.
3. Detection indicators
Body weight, clinical observation; Serum clinical chemical indicators, such as liver synthetic reserve function (ALB and CHE), liver injury (ALT, AST, ALP alkaline phosphatase, etc.) and renal function (creatinine, urea nitrogen, etc.); Hematology and blood biochemical analysis, such as white blood cell count, red blood cell count, hemoglobin concentration, blood sugar level, etc. Major organs (especially liver, kidney, heart) were evaluated histopathologically by tissue section staining; Immunohistochemical techniques, or immunohistochemical techniques, assess whether a drug causes an inflammatory response or other cellular immune response.
(3) In vivo pharmacological efficacy experiment
1. Experimental model
Humanized mouse models of drug target genes (hPCSK9, hGCGR, hGLP1R, hPD-1, hPD-L1, etc.), Hu-URG® and HBV-Tg.
2. Experimental design
Mice were treated with special diet or other methods (such as hepatitis B virus infection) to construct disease models and then given drug intervention to observe the changes of disease indicators. After intervention, knockdown efficiency of target genes and expression levels of upstream and downstream associated genes were detected.
3. Detection indicators
Body weight, blood lipids, liver function (ALB, CHE, ALT, AST, ALP, etc.), kidney function (creatinine, urea nitrogen, etc.), protein expression in blood (Elisa kit); Gene expression levels in tissues and organs (q-PCR, WB or Elisa kit, etc.); Histopathological tests (H&E, IHC, etc.); Virus infection (HBV DNA, HBsAg, HBeAg) and other indicators.
Case 1: The role of the siRNA drug Inclisiran in hPCSK9 mice

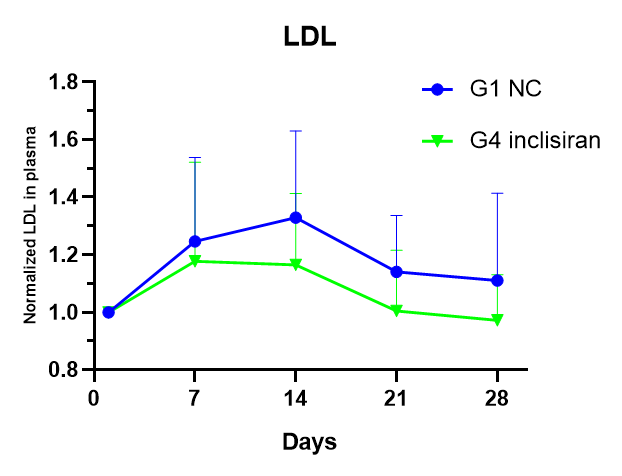
Figure 1. The role of Inclisiran in WD-induced hPCSK9 mice (n=8).
Note: At left, inclisiran reduces serum levels of hPCSK9 protein in hPCSK9 mice; At right, a Western diet (WD) upregates serum low-density lipoprotein (LDL) levels in male mice, and treatment with inclisiran reduces serum LDLR levels.
The results showed that B6-hPCSK9 mice were induced by WD diet for 8 weeks, which was consistent with the characteristics of hyperlipidemia mice. A single intraperitoneal injection of the siRNA drug Inclisiran, at a dose of 20 mg/kg, was administered and blood samples were taken for 4 weeks after administration, which consistently showed a reduction in blood hPCSK9 protein and LDL after administration.
Case 2: Application of HBV-Tg mice in preclinical small nucleic acids drug efficacy evaluation[1]
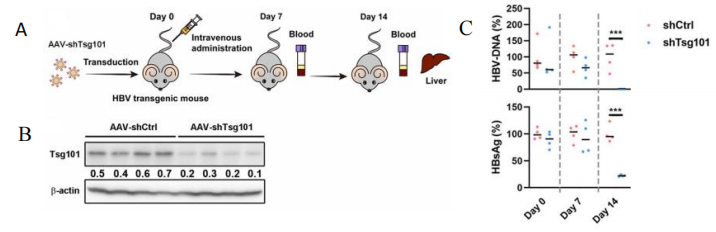
Fig 2. Effect of AAV-shTsg101 targeting Tsg101 on HBV particle secretion.
Eight 6-8 week-old B6N-Tg(1.28mer HBV)/Vst(genotype A) mice were randomly divided into two groups. shRNA or AAV-shTsg101 was administered through a caudal vein. Serum HBsAg and HBV DNA levels were measured at 7 and 14 days after initial administration. Tsg101 levels in the liver were measured 14 days after the first dose. The results showed that TSG101 knockdown can significantly inhibit serum HBsAg and HBV DNA in HBV-TG mice, confirming that TSG101 recognizes ubiquitinating HBc protein and enables HBV to exject via MVB pathway, providing a new target for antiviral drug development.
Reference
1. Zheng Y, et al. Hepatitis B virus hijacks TSG101 to facilitate egress via multiple vesicle bodies. PLoS Pathog. 2023, 19(5):e1011382.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167