Xenograft models play a significant role in cell and gene therapy research, particularly in the screening of candidate drugs, assessment of new therapies, and acceleration of drug development. Xenograft models typically involve transplanting human cells or tissues into immunodeficient mice to study the behavior and therapeutic responses of these human cells or tissues in vivo. Validation of cell and gene therapies in xenograft models provides valuable reference for the clinical trials of new therapies. Despite technical challenges and some limitations, these models hold great potential in advancing regenerative medicine, cancer research, and personalized treatment. By carefully designing experiments and integrating a variety of models, clinically relevant research outcomes can be obtained, providing important basis for the development of future treatment strategies.
We have established xenograft models, CDX or PDX, using highly immunodeficient mice to verify the efficacy of various cell and gene therapies, involving animals such as NPG, HSC-NPG, PBMC-NPG, PBMC-DK-NPG, HSC-GM3-NPG mice, as well as F344RG rats. Below are a few typical examples.
1. NPG-CDX mouse NK cell therapy[1]
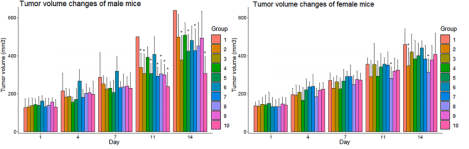
Fig1. Tumor growth curves after NK cell treatment in NPG-CDX mice
7-8-week-old NPG mice (n=100, 50 male and female) were subcutaneously injected with 0.2 mL of logarithmic growth phase BxPC-3 cell suspension (2.75×107 cells/mL; cell viability of 97%) to establish a subcutaneous tumor-bearing mouse xenograft model. 8 days later, 10 groups were randomly divided into 10 groups according to sex, and the average tumor volume of each group ranged from 20-200 mm3, and different sites were injected with different numbers of NK cells. Group 1 were injected intravenously with sodium chloride as a carrier control, groups 2-4 were injected intravenously with human peripheral blood-derived NK cells, groups 5-7 were injected intraperitoneally with NK cells, and groups 8-10 were injected with intratumorally injected NK cells, and the therapeutic effects were observed. The results showed that intravenous injection was the safest method to give NK cells to mice carrying human PaC, and had obvious tumor suppression effects on tumors.
2. NPG mice constructed for human colorectal cancer (NPG-PDX) and HER2-specific CAR-T cell therapy[2]
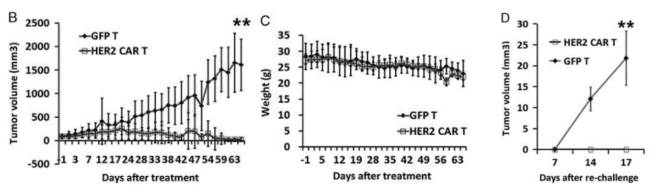
Fig 2. Construction of the NPG-PDX model and the antitumor effects of HER2 (Human Epidermal Growth Factor Receptor 2)-specific CAR-T cells.
6-10 week-old NPG mice (n=6 per group) were subcutaneously inoculated with tumor fragments (CRC) from colorectal cancer patients and infused with 5×106 T cells on days 7 and 14. P1 xenograft tumor tissues were re-transplanted on day 46. In this experiment, P1 and P2 generations of PDX models were used, and when the tumor volume reached 50-100 mm3, 2×106 GFP-T cells or HER2-specific CAR-T cells were intravenously injected into the tumor-bearing mice. The results showed that after 2 months of treatment with HER2-specific T cells, the tumors in NPG-PDX mice were completely eliminated, and the body weight growth parameters were not significantly different from those of the untreated control group.
3. NPG-CDX model for human lymphoma and DKO CAR-T cell gene therapy[3]
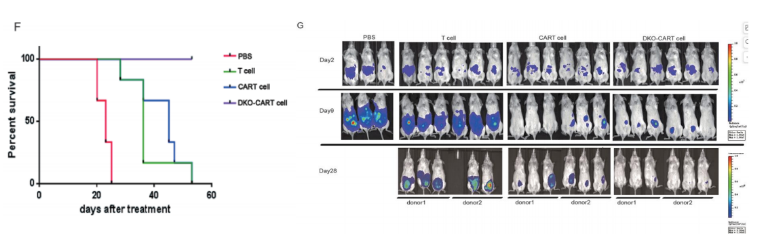
Fig 3. Tumor growth curves after gene therapy with DKO CAR-T cells in NPG-CDX mice
6-12 week-old NPG mice were intraperitoneally injected with 2×105 Raji-ffluc cells (human lymphoma). Tumor engraftment was assessed by imaging two days later. Mice were grouped based on tumor burden, and one day later were intraperitoneally injected with 200 μL PBS, 5×106 T cells, 5×106 CAR-T cells, or 5×106 DKO CAR-T (TRAC/B2M knockout) cells. The antitumor effects at different times were observed in each group. The results showed that the tumors in mice treated with standard CAR-T cells and DKO-CAR-T cells were significantly smaller than those in the unmodified T cells or PBS group, and the survival time was also longer.
4. NPG-PDX model for human colorectal cancer and CISR-CAR T cell immunotherapy[4]
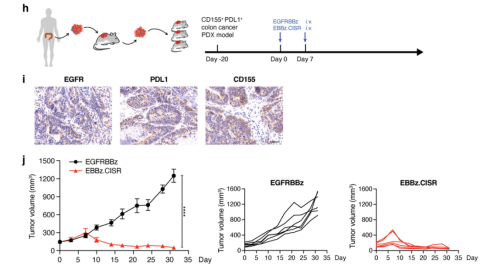
Figure 4. Construction of NPG-PDX mice and the effect of CISR-CAR T cell immunotherapy.
Colorectal cancer PDX tumor tissues were subcutaneously transplanted into NPG mice (n=6 per group). Approximately 20 days after implantation, when the tumor volume reached 100-200 mm3, the expression profiles of EGFR, PDL1, and CD155 in the tumor tissues used to establish the PDX model were analyzed using immunohistochemical staining. Mice were administered two intravenous injections of 3×106 EGFRBBz-CAR-T cells or EBBz.CISR T cells to observe the therapeutic effects. The results indicated that the established colorectal cancer PDX model was able to detect the markers EGFR, PDL1, and CD155, and that EBBz.CISR T cells had significant tumor-suppressing effects.
Reference
[1] Huang L, Lyu Z, Yang H, et al. Acute toxicities of intravenous, intraperitoneal, or intratumoral injection of natural killer cells in human pancreatic adenocarcinoma-bearing mice: Randomized study. Int Immunopharmacol. 2023, 124(Pt A):110881.
[2] Teng R, Zhao J, Zhao Y, et al. Chimeric Antigen Receptor-modified T Cells Repressed Solid Tumors and Their Relapse in an Established Patient-derived Colon Carcinoma Xenograft Model. J Immunother. 2019, 42(2):33-42.
[3] Liu X, Zhang Y, Cheng C, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017, 27(1):154-157.
[4] Zhao J, Dong J, Deng C, et al. Enhancing T cell anti-tumor efficacy with a PD1-TIGIT chimeric immune-checkpoint switch receptor. Oncoimmunology. 2023,12(1):2265703.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167