Liver injury is a critical early step in a variety of liver diseases. By studying the process of liver injury, it is possible to identify and diagnose liver diseases earlier, thereby preventing the disease from progressing to more severe conditions such as fibrosis or cirrhosis. Therefore, in-depth research on the mechanisms of liver injury and fibrosis is not only conducive to understanding the occurrence and development of liver diseases but also provides a key theoretical basis for early diagnosis, disease monitoring, and treatment, aiding in the development of new drugs and personalized treatment plans. VitalStar has developed adjustable mouse models of liver injury, including URG, NPG-Fah, and B6.Tet-UPA, B6-Fah, as well as HBV-Tg+CCL4 models of liver fibrosis and cirrhosis. These models have served numerous CRO and research clients in drug screening, efficacy evaluation, and toxicity research, contributing to new drug development and personalized treatment regimens.
The non-infectious liver disease models Vitalstar could provide include:
Models | Species | Characteristics of Models | |||||
Body Weight | Fast Blood Glucose | Steatosis | Hepatic Inflammation | Hepatic Fibrosis | Hepatocellular Carcinoma (HCC) | ||
HFD | Mouse/Rat | Increase | Elevated | 8-16 W for mice 8-24 W for rats | 12-24 W | Mild | No |
GAN NASH | Mouse/Rat | Increase | Elevated | 10-12 W | 12-16 W | Moderate, ≥12 M | No |
MCD | Mouse/Rat | Decrease | Reduced | Mild, 4 W | Mild, 4 W | 4-6 W | No |
CDAHFD | Mouse | Unchanged | Elevated | 8-12 W | 8-12 W | 8-12 W | 24-36 W, 100% |
HBV-Tg+CCl4 | Mouse | Increase | Unchanged | No | 4-6 W | 6-8 W | 24 W |
HFD+CCl4 | Mouse | Increase | Elevated | 8-12 W | 8-12 W | F3, 16 W | 24 W |
Alb/Pten KO | Mouse | Unchanged | Unchanged | No | No | 10-40 W | 70-78 W, 100% |
Alms1 KO+WD | Mouse | Increase | Elevated | 4 W | 4 W | F3, 16 W | 24 W, 75% |
Ldlr KO+WD | Mouse | Increase | Elevated | 8-12 W | 8-12 W | 8-12 W | 24 W, 100% |
Hu-URG+CDAHDF | Humanized liver | Unchanged or Slightly Decreased | Elevated | 4-8 W | 4-8 W | 4-12 W | 20-24 W |
Based on these liver disease models, Vitalstar provides customized animal models for liver damage, fibrosis, cirrhosis, and hepatocellular cancer researches and preclinical pharmacology and efficacy services. The related detections and analysis that VitalStar can carry out include:
1. Serum testing: such as ALT, AST, ALP, TBil, ALB, etc. (blood biochemistry) or other serum markers (ELISA).
2. Liver tissue testing: α-SMA, Col1, TGFβ1, etc. (by immunohistochemistry, Western blot or ELISA, RT-PCR, etc.).
3. Pathological analysis: pathological section, HE staining, Sirius Red staining, Masson staining, immunohistochemistry, and pathological reading.
(1)Using URG® mice to evaluate hepatocyte-like cell (HLC) transplantation treating liver failure [1]
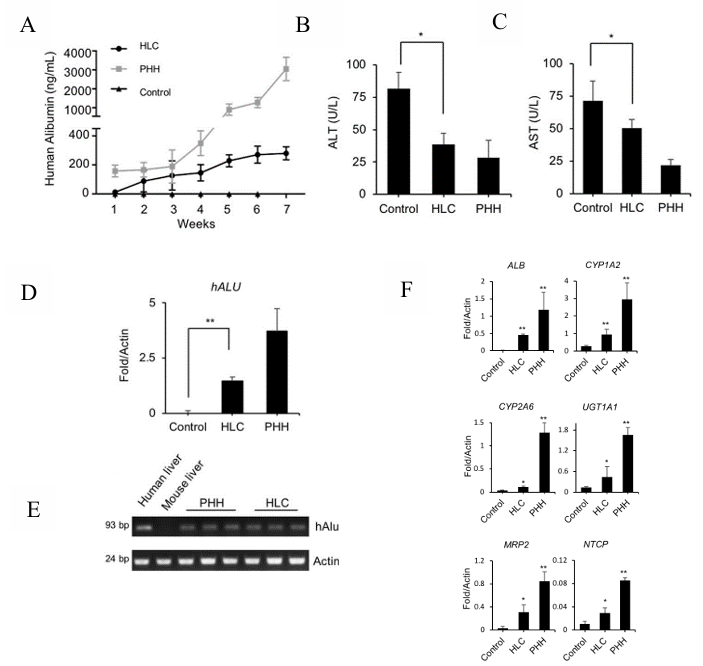
Figure 1. Regeneration of livers in URG® mice after HLC transplantation
Male URG mice at 8 weeks of age (n=5/group) were administered doxycycline (Dox) at a dose of 15 mg/kg body weight via intraperitoneal injection 24 hours prior to cell transplantation. Subsequently, hepatocyte-like cells (HLCs, 2×10^6 cells) and primary human hepatocytes (PHHs, 2×10^6 cells) were transplanted, with PBS (n=5) as control, via intrasplenic injection. The URG® mice were continuously fed with Dox-supplemented drinking water for 6 weeks post-cell transplantation. Blood sample was collected weekly from the tail vein, and human albumin (ALB) levels were quantitatively measured by ELISA. The mice were euthanized at week 7 and subject to examination. Results indicated that after transplantation of HLCs into URG® mice, the secretion of human ALB in the mouse serum gradually increased. After 7 weeks, the serum concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were significantly reduced. Real-time quantitative PCR for human-specific Alu sequences further confirmed the engraftment of HLCs in the mouse liver. The expression of human-specific genes such as ALB, and human hepatocyte-specific metabolic genes of phase I enzymes CYP1A2 and CYP2A6 in the chimeric liver tissue were also detected. These data suggest that HLCs can integrate into the livers of URG® mice, leading to an improvement in liver dysfunction.
(2)NPG-Fah mice as liver injure models
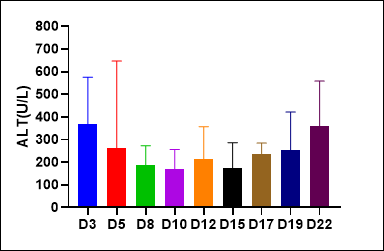
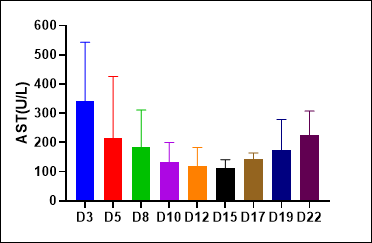
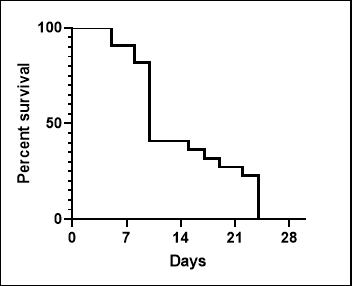
Figure 2. Liver injury indicators ALT and AST levels in serum of NPG-Fah mice and survival rate after withdrawing of NTBC
(3)B6.Tet-uPA mice as liver injury models
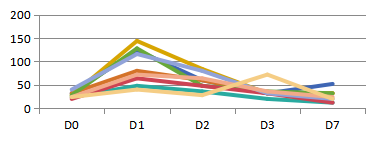
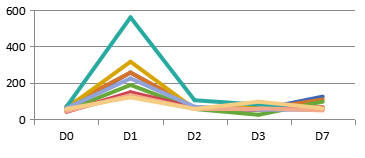
A:ALT(IU/mL) B:AST(IU/mL)
Figure 3. Liver injury indicators ALT and AST levels in serum of B6.Tet-uPA mice after ip. injection of Dox(100mg/kg)
(4)HBV-Tg+CCL4 liver fibrosis and cirrhosis mouse model[2]
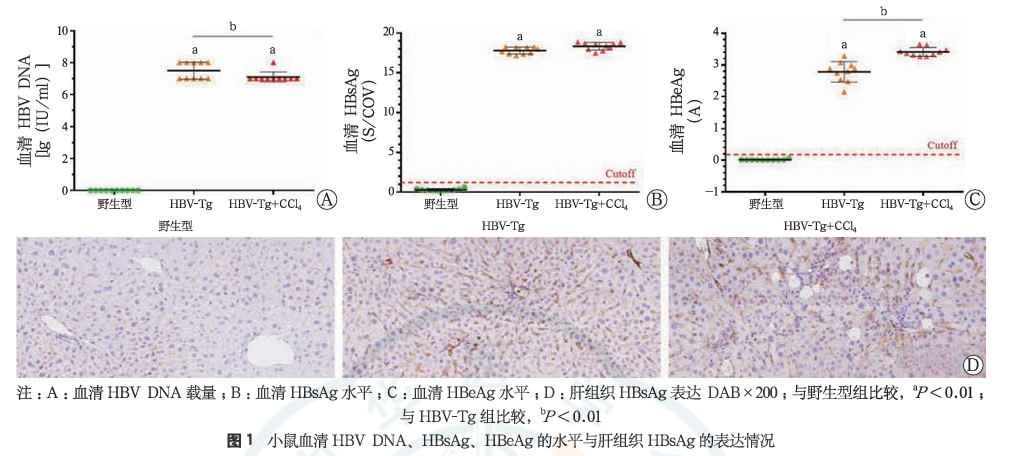
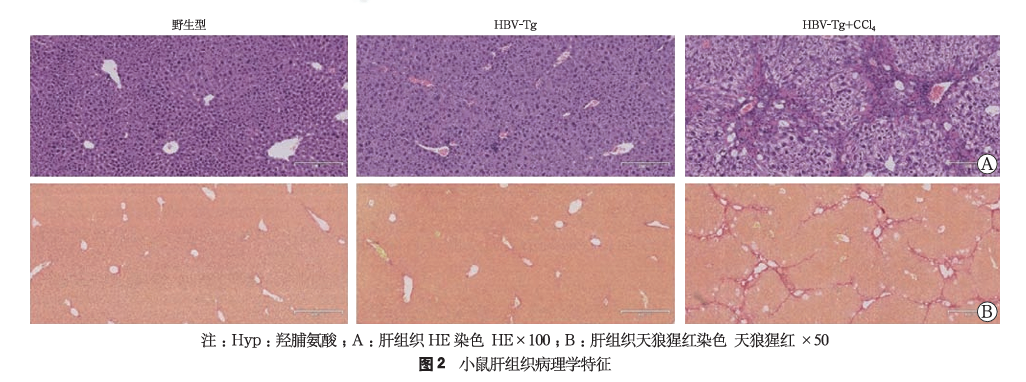
20 male HBV-Tg mice were randomly divided into two groups (simple HBV-Tg group and HBV-Tg combined with CCL4 model group), with 10 mice in each group; at the same time, 10 wild-type mice were used as a control group. After one week of adaptive breeding, the HBV-Tg combined with CCL4 model group was administered with 10% CCL4-olive oil solution intraperitoneal injection (2 mL/kg, every other day, for a total of 6 weeks to induce fibrosis), while the other two groups were given the same frequency and volume of olive oil intraperitoneal injections. The results showed that the HBV-Tg mice combined with CCL4 intraperitoneal injection induced a mouse model of liver fibrosis with a background of hepatitis B, characterized by positive HBsAg and HBeAg, and high expression of HBV DNA; hepatocytes were disordered, the structure of the liver lobules was destroyed, there was a large amount of inflammatory cell infiltration in the portal areas, hepatocyte swelling and necrosis, collapse of the reticular framework, and obvious fatty changes and ballooning (Figure 2A); a large amount of collagen fibers were deposited in the portal areas and extended around, forming distinct fibrous septa.
References:
1. Li Z, Wu J, Wang L, et al. Generation of qualified clinical-grade functional hepatocytes from human embryonic stem cells in chemically defined conditions. Cell Death Dis. 2019, 10:763.
2. 孙鑫, 黄凯, 张满, 等. HBV-Tg复合四氯化碳模型小鼠肝内淋巴细胞亚群变化特点及其与HBV病毒学和肝纤维化的相关性分析. 中华肝病杂志. 2020, 28:580-585.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167