The metabolic characteristics and regularities of drugs after entering the human body are directly related to the drug's efficacy, effective dosage, and potential side effects. Therefore, pharmacokinetic research is an essential part of new drug development. Preclinical in vitro and in vivo pharmacokinetic studies of new drugs can provide important references for clinical trial dosage forms, dosages, dosing cycles, and safety toxicology.
Liver is the primary organ for drug metabolism. Due to the species differences in cytochrome P450 families and transporter proteins etc. related to drug metabolism between animals and human, the metabolic pathways and metabolites of drugs in animals and humans are not the same. Therefore, the pharmacokinetic characteristics obtained from animal experiments in preclinical pharmacokinetic study may not accurately reflect that in human body. In addition to pharmacokinetic characteristics, there may also be different manifestations in drug interactions, detoxification, and the production of toxic metabolites. The use of human hepatocytes and humanized liver animal models for in vitro and in vivo research on drug metabolism can greatly overcome the shortcomings of animal models and obtain better clinically relevant research results.
Hu-URG® is a humanized liver mouse model established by transplanting primary human hepatocytes into URG mice with inducible liver injury, developed by Vitalstar Company. The replacement rate of human hepatocytes can reach 90%, which means human hepatocytes become the mainstream in the chimeric mouse liver while maintaining the normal functions of human hepatocytes, expressing genes related to drug metabolism. In practical applications, Hu-URG® exhibits drug metabolic characteristics that are different from mice but close to humans, making it a very suitable model for studying the metabolism of new drugs in vivo. In addition, Vitalstar Company has primary human hepatocyte (PHH) resource that can be used for in vitro drug metabolism studies, and can provide compliant in vitro pharmacokinetic study services based on PHH.
(1) Using Hu-URG® mice to do drug metabolism study
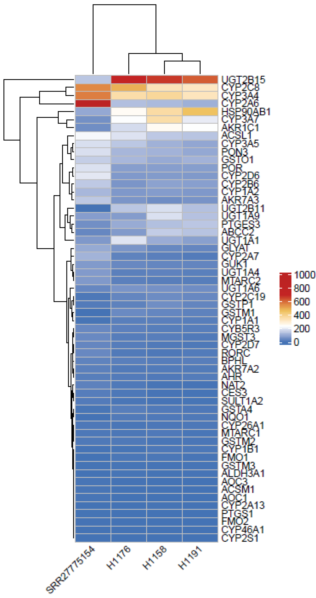
Figure 1. Comparison of liver transcriptomes between Hu-URG® mice and human
The transcriptomes of Hu-URG® mouse livers(from H1176, H1158 and H1191, 3 Hu-URG® mice)are similar to that of humans(SRR27775154).
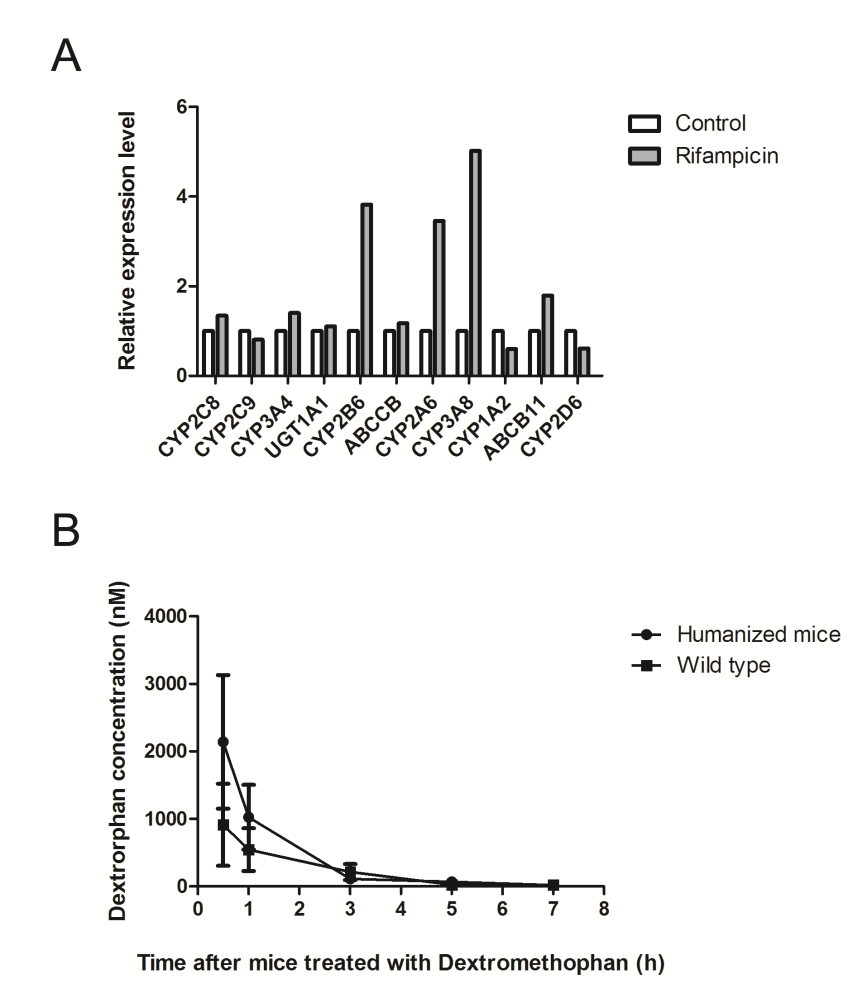
Figure 2. Hu-URG® mice manifest metabolic characteristics different from mouse but more like human after administration of Rifampicin and Dextromethophan.
A: Rifampicin induced the human liver enzymes (CYP2B6, CYP2A6, CYP3A8, ABCB11, etc.) up-regulating in Hu-URG® mouse livers.
B: Dextromethophan manifests different metabolic characteristics in Hu-URG® and non-transplanted URG® mice.
Ketoprofen exhibits distinct metabolic characteristics in Hu-URG® mice compared to URG® mice that have not been engrafted with human hepatocytes.
(2) Using PHH to do drug metabolism study
Primary human hepatocytes (PHH) maintain many of their normal functions when cultured in adherent conditions, making them suitable for in vitro studies of drug metabolism. Vitalstar Company has extensive experience in the ex vivo culture of PHH and possess a P2 laboratory as well as PHH resources, enabling it to conduct compliant in vitro pharmacokinetic research services based on PHH.
Figure 3. Adherently cultured PHHs.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167