Xenograft tumor models have significant research and clinical value in the development of macromolecular drugs. By conducting efficacy assessments, safety studies, tumor microenvironment research, resistance mechanism studies, and exploration of personalized medicine within these models, the development and clinical translation of both large and small molecule drugs can be accelerated, providing new options and strategies for cancer treatment. These models provide an important experimental platform for understanding the mechanisms of action of large and small molecule drugs, optimizing treatment regimens, and improving therapeutic outcomes.
Currently, our efficacy testing services for large and small molecule drugs cover a variety of types, including monoclonal antibodies, bispecific antibodies, fusion proteins, recombinant proteins, vaccines, cell therapy products, gene therapy products, and stem cell therapies. The animals involved include NPG, HSC-NPG, PBMC-NPG, PBMC-DK-NPG, HSC-GM3-NPG mice, as well as F344RG rats. The F344RG rat, independently developed by Weitongda, is a highly immunodeficient rat. Its larger size allows for a larger tumor sample load, enabling the acquisition of more tumor samples. The larger size also means a greater circulating blood volume, allowing for high-frequency blood withdrawal and more blood samples, while reducing the number of animals and improving data consistency. It also lowers the difficulty of surgical operations (orthotopic cancer). The many advantages make the F344RG rat a good model for pharmacodynamic evaluation (anticancer drugs, cardiovascular drugs, metabolic disease research, etc.), safety evaluation (acute or chronic toxicity, carcinogenicity, reproductive and developmental toxicity, immunotoxicity research, etc.), and pharmacokinetic evaluation (absorption, distribution, and kinetic studies, etc.). Below are some practical cases of monoclonal antibodies, bispecific antibodies, and protein molecules.
1. HSC-NPG-CDX gastric cancer model and PD-1 antibody drug therapy[1]
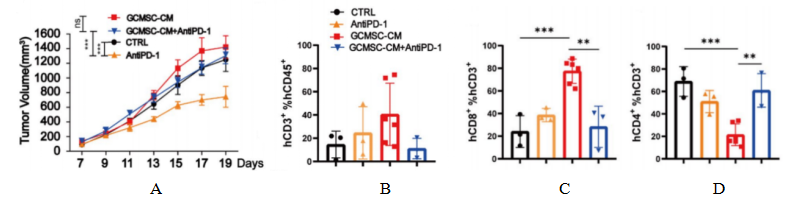
Fig1. Tumor and tumor tissue immune cell levels after HuHSC-NPG inoculation of gastric cancer tumors.
4-8 week-old NPG mice were irradiated with X-rays (1.2 Gy) and within 24 hours intravenously injected with 5×104 human umbilical cord blood CD34+ cells. After 12 weeks of reconstitution, patient-derived gastric cancer cell lines were inoculated, and treated with GCMSC-CM and PD-1 antibody alone or in combination to observe tumor changes, and to detect the levels of CD45+CD3+, CD3+CD8+, and CD3+CD4+ in tumor tissues from different treatment groups. The results showed that after 21 days of treatment, PD-1 antibody therapy significantly inhibited gastric tumor growth, while the combination of GCMSC-CM with PD-1 antibody significantly increased tumor volume compared to PD-1 antibody alone, indicating that GCMSC-CM negated the inhibitory effect of PD-1 antibody on gastric tumor growth.
2. NPG-CDX lung adenocarcinoma model and PD-L1xCD3 bispecific antibody drug therapy[2]
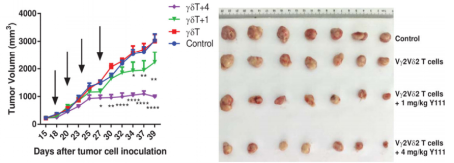
Fig2. The combined usage of transfused Vγ2V2d T cells with Y111 significantly inhibited tumor growth in vivo
6-8 week-old female NPG mice were s.c. inoculated with 5´106 H1975 NSCLC cells on Day 0. After seventeen days, mice were treated with i.v. transfused Vγ2Vδ2 T (1´106) cells w/wo 1 or 4 mg/kg Y111. These treatments were repeated twice per week for 2 weeks. Mice treated PBS were used as control. Results: Intravenous injection of Y111 (4 mg/kg) combined with Vγ2Vδ2 T cells significantly inhibited tumor growth in NPG mice.
3. NPG-CDX human prostate cancer model and plant protein antitumor efficacy[3]
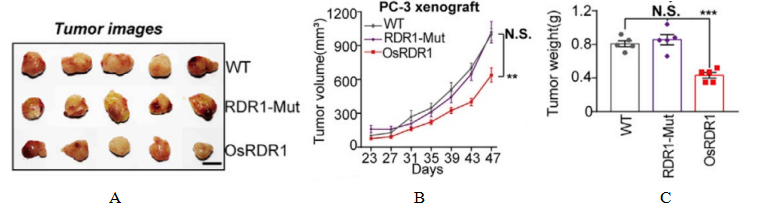
Fig3. Tumor effects of the plant immunoglobulin RDR1
4-6 week-old female NPG mice (n=5 per group) were subcutaneously implanted in the abdomen with RDR1-inducible 2106 PC-3 cells on day 0, and doxycycline (Dox) was administered in their drinking water to achieve ectopic expression of RDR1, with tumor growth status being monitored. The results indicated that the wild-type, but not the mutant, RDR1 significantly inhibited the size, volume, and weight of the resulting tumors.
4. F344RG rat CDX human glioma CAR-T drug
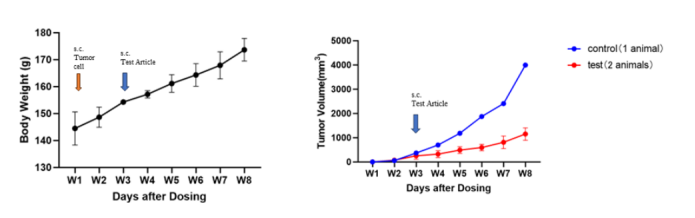
Fig4. The tumor-suppressing effect of CAR-T therapy on glioma
Reference
[1] Huang C, Chen B, Wang X, et al. Gastric cancer mesenchymal stem cells via the CXCR2/HK2/PD-L1 pathway mediate immunosuppression. Gastric Cancer. 2023, 26(5):691-707.
[2] Yang R, Shen S, Gong C, et al. Bispecific Antibody PD-L1 x CD3 Boosts the Anti-Tumor Potency of the Expanded Vγ2Vδ2 T Cells. Front Immunol. 2021,12:654080.
[3] Qi Y, Ding L, Zhang S, et al. A plant immune protein enables broad antitumor response by rescuing microRNA deficiency. Cell. 2022, 185(11):1888-1904.e24.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167