Viral hepatitis is major infectious diseases that affects human liver, caused by viruses such as HAV, HBV, HCV, HDV, and HEV. Except for HAV, none of them have routine small animal infection model. HDV is a satellite virus of HBV and must rely on the surface antigen protein encoded by the HBV genome to package into infectious viral particles.
Vitalstar has successfully established infection models for HBV, HCV, HDV, and HEV on its independently developed humanized liver mouse models, providing mouse models for these hepatitis viruses. We possess ABSL-2 laboratory, capable of undertaking in vivo and in vitro virology research and anti-viral drug screening and evaluation services.
In addition to the Hu-URG® mouse model that can simulate the entire life cycle of HBV infection, Vitalstar has also developed an HBV transgenic (type A) mouse model that produces high titers of HBV viremia, with HBV DNA content reaching 1×108 IU/mL, HbsAg content above 103 IU/mL, and HBeAg content above 102 IU/mL. Furthermore, Vitalstar has established AAV vector-mediated HBV infection mouse models (types B, C, D). These HBV-carrying models, established on immunocompetent mice (such as C57BL/6), produce long-term stable high titers of HBV viral particles and antigens, which can be used for the screening and evaluation of anti-HBV drugs, including immunotherapeutic treatments.
Vitalstar also has HBV-expressing cell lines, NTCP-HepG2 cell line and primary human hepatocyte stockage which support HBV infecting, capable of providing in vitro evaluation services for anti-HBV drug efficacy studies.
In Vivo pharmacological study of anti-HBV drugs
1. Virus resource
To establish HBV infection mouse models or cell cultures, Vitalstar maintains a reserve of active HBV virus isolates, including genotype A (derived from serum of HBV transgenic mice), genotypes B and C (derived from patient serum and amplified on Hu-URG mice), as well as genotype D (concentrated from the culture supernatant of HepAD38 cells).
2. HBV indicator test
Vitalstar possesses a registered Biosafety Level 2 laboratory and animal facility, equipped with sophisticated instruments for HBV indicator detection and analysis, as well as rigorous and skilled laboratory personnel and has experience in IND (Investigational New Drug) application submissions. Leveraging its leading HBV animal models and related bioanalytic competence, Vitalstar is capable of undertaking pharmacological and efficacy research services for new anti-HBV drugs, including small molecules, antibodies, small rebonucleic acids, immunotherapeutics, and traditional Chinese medicine.
For anti-HBV drug efficacy studies, the examine items include:
(1) HBV DNA and HBV RNA detection (quantitative PCR method).
(2) Quantitative detection of HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb (electrochemiluminescence method).
(3) Relative quantitative detection of HBsAg, HBeAg, and HBcAg contents in liver tissue (ELISA method) or immunohistochemical assays.
(4) Detection of HBV cccDNA in liver tissue (quantitative PCR method).
(5) Evaluation of immune therapeutic effects or immune reactivation to hepatitis B using intracellular staining flow cytometry (ICS) and ELISPOT assays.
3. Study Cases using serial of HBV infection mouse models
(1) Viral indicators in serum of HBV infected Hu-URG® mice and efficacy data of anti-HBV drug

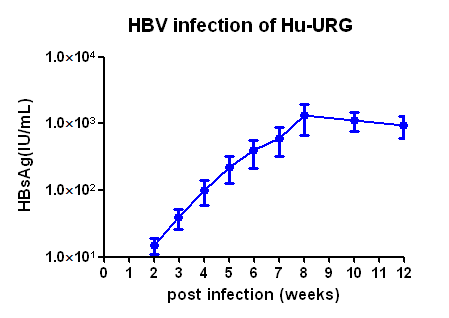
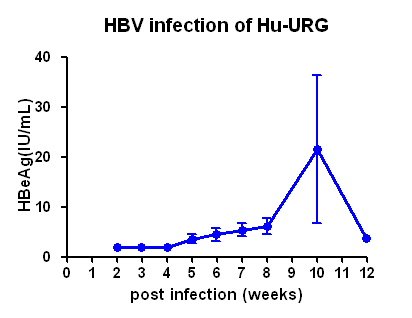
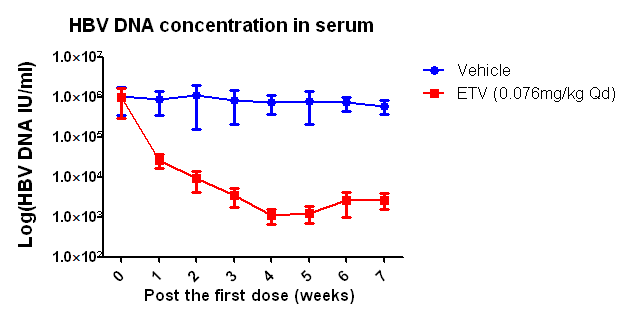
Figure 1. HBV infection dynamics in Hu-URG® mice and anti-HBV efficacy of ETV
(2) HEV infection data in Hu-URG® mice [1]
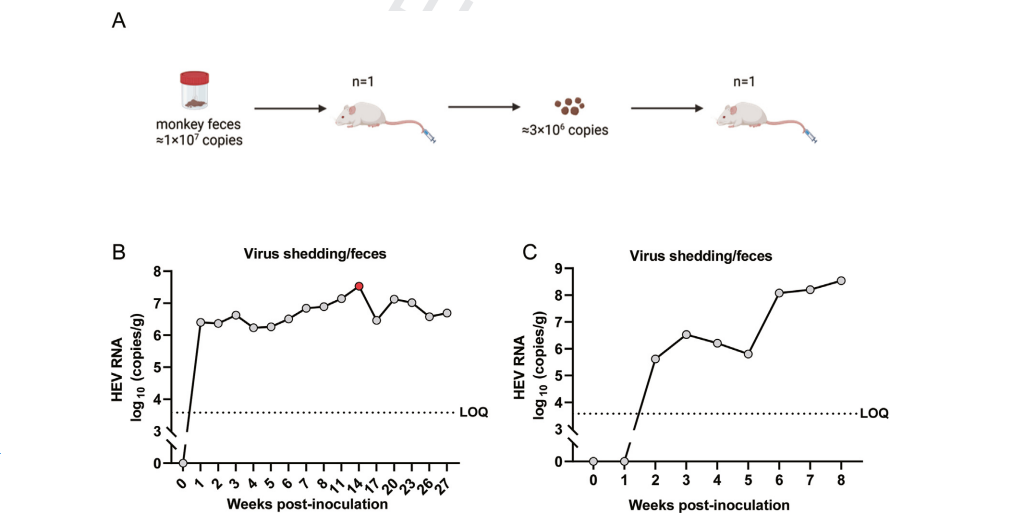
Figure 2. Infection and passage of HEV-1 in Hu-URG® mice and quantitative analysis of HEV RNA load in fecal samples.
(3) Viral indicators in AAV-HBV mice
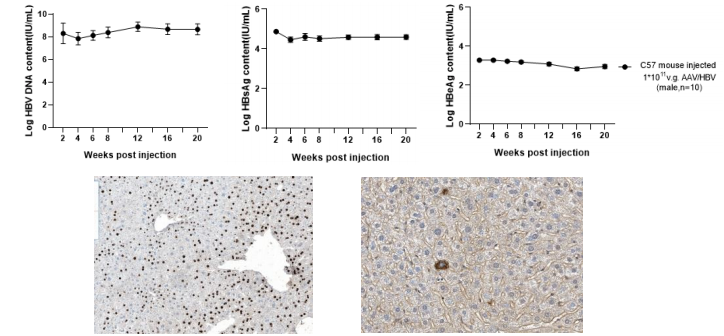
Figure 3. Dynamics of viral indicators in serum and immunohistochemistry stain of HBcAg and HBsAg in liver of AAV-HBV type D mice
(4) Study of anti-HBV activity of polysaccharides on HBV-Tg mice[2]
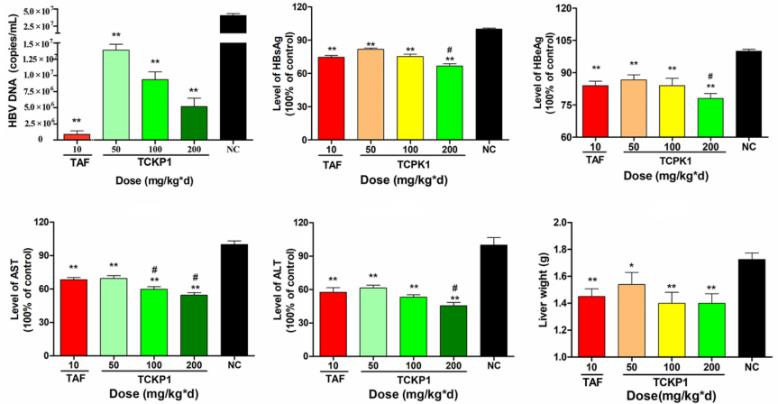
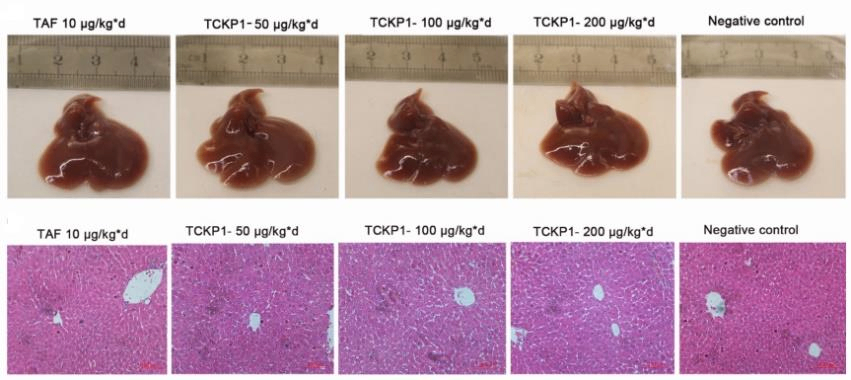
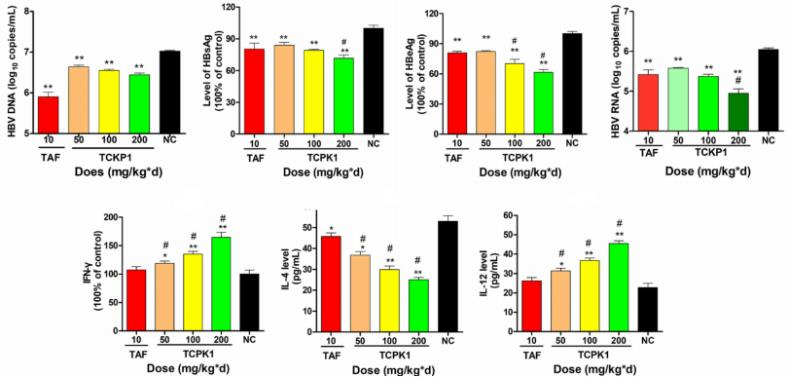
A B C
Figure 4. anti-HBV activity of TCKP1
In vitro anti-HBV pharmacological and efficacy study
1. For in vitro pharmacological and efficacy study of anti-HBV drugs, Vitalstar also has a mature platform and technical team. The cell lines or primary cells currently available in Vitalstar include:
(1) Constant HBV expression cell lines: HepG2.2.15, HepAD38
(2) HBV susceptible cells: HepG2-NTCP cell line, PHH
(3) HBV resource: isolate genotypes include Type A (derived from the serum of HBV transgenic mice),Type B and C (derived from patient serum and expanded in Hu-URG® mice) and Type D (concentrated from HepAD38 cell culture supernatant).
2. Available detection and analysis items:
(1) HBsAg, HBeAg and HBcAg contents (in supernatant or cell lysate) by immunohistochemical assays
(2) HBV DNA, cccDNA and HBV RNA detection (quantitative PCR method)
Reference:
1. Liu T, He Q, Yang X, et al. An Immunocompetent Mongolian Gerbil Model for Hepatitis E Virus Genotype 1 Infection. Gastroenterology. 2024, 167(4):750-763.e10.
2. Tang F, Huang G, Lin L, et al. Anti-HBV Activities of Polysaccharides from Thais clavigera (Küster) by In Vitro and In Vivo Study. Mar Drugs. 2021, 19(4):195.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167